Clinical characteristics, treatment patterns and relapse in patients with clinical stage IS testicular cancer
- PMID: 34854948
- PMCID: PMC8921055
- DOI: 10.1007/s00345-021-03889-x
Clinical characteristics, treatment patterns and relapse in patients with clinical stage IS testicular cancer
Abstract
Purpose: Clinical stage I (CSI) testicular germ cell tumors (TGCT) represents disease confined to the testis without metastasis and CSIS is defined as persistently elevated tumor markers (TM) after orchiectomy, indicating subclinical metastatic disease. This study aims at assessing clinical characteristics and oncological outcome in CSIS.
Methods: Data from five tertiary referring centers in Germany were screened. We defined correct classification of CSIS according to EAU guidelines. TM levels, treatment and relapse-free survival were assessed and differences between predefined groups (chemotherapy, correct/incorrect CSIS) were analyzed with Fisher's exact and Chi-square test.
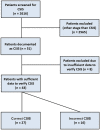
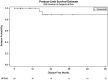
Results: Out of 2616 TGCT patients, 43 (1.6%) were CSIS. Thereof, 27 were correctly classified (cCSIS, 1.03%) and 16 incorrectly classified (iCSIS). TMs that defined cCSIS were in 12 (44.4%), 10 (37%), 3 (11.1%) and 2 (7.4%) patients AFP, ß-HCG, AFP plus ß-HCG and LDH, respectively. In the cCSIS group, six patients were seminoma and 21 non-seminoma. Treatment consisted of active surveillance, carboplatin-mono AUC7 and BEP (bleomycin, etoposide and cisplatin). No difference between cCSIS and iCSIS with respect to applied chemotherapy was found (p = 0.830). 5-year relapse-free survival was 88.9% and three patients (11%) in the cCSIS group relapsed. All underwent salvage treatment (3xBEP) with no documented death.
Conclusion: Around 1% of all TGCT were classified as cCSIS patients. Identification of cCSIS is of critical importance to avoid disease progression and relapses by adequate treatment. We report a high heterogeneity of treatment patterns, associated with excellent long-term survival irrespective of the initial treatment approach.
Keywords: Chemotherapy; Clinical stage IS; Non-seminoma; Seminoma; Tumor markers.
© 2021. The Author(s).
Conflict of interest statement
The authors declare they have no financial or competing interests.
Figures
References
-
- Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, D.K.) (2019) AWMF, S3-Leitlinie Diagnostik, Therapie und Nachsorge der Keimzelltumoren des Hodens. 2019 (Langversion 1.0)
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical