Clostridium perfringens Beta2 toxin forms highly cation-selective channels in lipid bilayers
- PMID: 34854958
- PMCID: PMC8827211
- DOI: 10.1007/s00249-021-01577-7
Clostridium perfringens Beta2 toxin forms highly cation-selective channels in lipid bilayers
Abstract
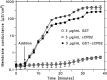
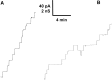
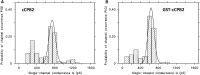
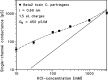
Clostridium perfringens is a potent producer of a variety of toxins. Well studied from these are five toxins (alpha, Beta (CPB), epsilon, iota and CPE) that are produced by seven toxinotype strains (A-G) of C. perfringens. Besides these toxins, C. perfringens produces also another toxin that causes necrotizing enterocolitis in piglets. This toxin termed consensus Beta2 toxin (cCPB2) has a molecular mass of 27,620 Da and shows only little homology to CPB and no one to the other toxins of C. perfringens. Its primary action on cells remained unknown to date. cCPB2 was heterogeneously expressed as fusion protein with GST in Escherichia coli and purified to homogeneity. Although cCPB2 does not exhibit the typical structure of beta-stranded pore-forming proteins and contains no indication for the presence of amphipathic alpha-helices we could demonstrate that cCPB2 is a pore-forming component with an extremely high activity in lipid bilayers. The channels have a single-channel conductance of about 700 pS in 1 M KCl and are highly cation-selective as judged from selectivity measurements in the presence of salt gradients. The high cation selectivity is caused by the presence of net negative charges in or near the channel that allowed an estimate of the channel size being about 1.4 nm wide. Our measurements suggest that the primary effect of cCPB2 is the formation of cation-selective channels followed by necrotic enteritis in humans and animals. We searched in databases for homologs of cCPB2 and constructed a cladogram representing the phylogenetic relationship to the next relatives of cCPB2.
Keywords: Cation-selectivity; Channel formation; Consensus Clostridium perfringens Beta2 toxin (cCPB2); Lipid bilayer membrane; Pore-forming toxins; Propidium iodide uptake.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no conflicts of interest or competing interests.
Figures





Similar articles
-
Clostridium perfringens Enterotoxin: The Toxin Forms Highly Cation-Selective Channels in Lipid Bilayers.Toxins (Basel). 2018 Aug 22;10(9):341. doi: 10.3390/toxins10090341. Toxins (Basel). 2018. PMID: 30135397 Free PMC article.
-
Clostridium perfringens delta toxin is sequence related to beta toxin, NetB, and Staphylococcus pore-forming toxins, but shows functional differences.PLoS One. 2008;3(11):e3764. doi: 10.1371/journal.pone.0003764. Epub 2008 Nov 19. PLoS One. 2008. PMID: 19018299 Free PMC article.
-
Clostridium perfringens beta-toxin forms potential-dependent, cation-selective channels in lipid bilayers.Infect Immun. 2000 Oct;68(10):5546-51. doi: 10.1128/IAI.68.10.5546-5551.2000. Infect Immun. 2000. PMID: 10992452 Free PMC article.
-
The enteric toxins of Clostridium perfringens.Rev Physiol Biochem Pharmacol. 2004;152:183-204. doi: 10.1007/s10254-004-0036-2. Epub 2004 Oct 23. Rev Physiol Biochem Pharmacol. 2004. PMID: 15517462 Review.
-
Mechanisms of Action and Cell Death Associated with Clostridium perfringens Toxins.Toxins (Basel). 2018 May 22;10(5):212. doi: 10.3390/toxins10050212. Toxins (Basel). 2018. PMID: 29786671 Free PMC article. Review.
Cited by
-
Clostridium perfringens in central Colombia: frequency, toxin genes, and risk factors.Gut Pathog. 2024 Jul 4;16(1):32. doi: 10.1186/s13099-024-00629-5. Gut Pathog. 2024. PMID: 38965598 Free PMC article.
-
How to isolate channel-forming membrane proteins using the E. coli expression system.Nat Protoc. 2025 Feb;20(2):462-479. doi: 10.1038/s41596-024-01055-2. Epub 2024 Oct 4. Nat Protoc. 2025. PMID: 39367089 Review.
-
Unveiling the pathogenic mechanisms of Clostridium perfringens toxins and virulence factors.Emerg Microbes Infect. 2024 Dec;13(1):2341968. doi: 10.1080/22221751.2024.2341968. Epub 2024 Apr 27. Emerg Microbes Infect. 2024. PMID: 38590276 Free PMC article. Review.
-
Identification and Characterization of Clostridium perfringens Atypical CPB2 Toxin in Cell Cultures and Field Samples Using Monoclonal Antibodies.Toxins (Basel). 2022 Nov 17;14(11):796. doi: 10.3390/toxins14110796. Toxins (Basel). 2022. PMID: 36422970 Free PMC article.
-
Peptide-based pore formation and cell membrane deformation: European Biophysics Journal Prizes at EBSA 2023.Eur Biophys J. 2023 Nov;52(8):619-623. doi: 10.1007/s00249-023-01691-8. Eur Biophys J. 2023. PMID: 37994943
References
-
- Allaart JG, de Bruijn ND, van Asten AJ, Fabri TH, Gröne A (2012) NetB-producing and beta2-producing Clostridium perfringens associated with subclinical necrotic enteritis in laying hens in the Netherlands. Avian Pathol 41(6):541–546 - PubMed
-
- Allaart JG, van Asten AJ, Vernooij JC, Gröne A (2014) Beta2 toxin is not involved in in vitro cell cytotoxicity caused by human and porcine cpb2-harbouring Clostridium perfringens. Vet Microbiol 171(1–2):132–138 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

