Value appropriation in hepatitis C
- PMID: 34855072
- PMCID: PMC9304061
- DOI: 10.1007/s10198-021-01409-7
Value appropriation in hepatitis C
Abstract
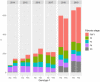
Background: In 2015, the Swedish government in an unprecedented move decided to allocate 150 million € to provide funding for new drugs for hepatitis C. This was triggered by the introduction of the first second generation of direct-acting antivirals (DAAs) promising higher cure rates and reduced side effects. The drugs were cost-effective but had a prohibitive budget impact. Subsequently, additional products have entered the market leading to reduction in prices and expansions of the eligible patient base.
Methods: We estimated the social surplus generated by the new DAAs in Stockholm, Sweden, for the years 2014-2019. The actual use and cost of the drugs was based on registry data. Effects on future health care costs, indirect costs and QALY gains were estimated using a Markov model based primarily on Swedish data and using previous generations of interferon-based therapies as the counterfactual.
Results: A considerable social surplus was generated, 15% of which was appropriated by the producers whose share fell rapidly over time as prices fell. Most of the consumer surplus was generated by QALY gains, although 10% was from reduced indirect costs. QALY gains increased less rapidly than the number of treated patients as the eligibility criteria was loosened.
Conclusions: The transfer of funds from the government to the regions helped generate substantial surplus for both consumers and producers with indirect costs playing an important role. The funding model may serve as a model for the financing of innovative treatments in the future.
Keywords: Consumer surplus; Cost-effectiveness; Hepatitis C; Pharmaceutical reimbursement; Producer surplus.
© 2021. The Author(s).
Conflict of interest statement
PL, SL and GB are employed by the Swedish Institute for Health Economics, an independent research organization with work funded by different stakeholders in health care, including manufacturers of hepatitis C drugs. OW and BJ reports consultancies and speakers’ bureaus with AbbVie, BMS, Gilead and MSD/Merck.
Figures


Similar articles
-
Cost-utility analysis of second-generation direct-acting antivirals for hepatitis C: a systematic review.Expert Rev Gastroenterol Hepatol. 2018 Dec;12(12):1251-1263. doi: 10.1080/17474124.2018.1540929. Epub 2018 Oct 31. Expert Rev Gastroenterol Hepatol. 2018. PMID: 30791790
-
Cost-effectiveness and budget impact of interferon-free direct-acting antiviral-based regimens for hepatitis C treatment: the French case.J Viral Hepat. 2016 Oct;23(10):767-79. doi: 10.1111/jvh.12546. Epub 2016 May 4. J Viral Hepat. 2016. PMID: 27144512
-
Assessment of cost of innovation versus the value of health gains associated with treatment of chronic hepatitis C in the United States: The quality-adjusted cost of care.Medicine (Baltimore). 2016 Oct;95(41):e5048. doi: 10.1097/MD.0000000000005048. Medicine (Baltimore). 2016. PMID: 27741116 Free PMC article. Review.
-
Cost-effectiveness of integrated treatment for hepatitis C virus (HCV) among people who inject drugs in Norway: An economic evaluation of the INTRO-HCV trial.Addiction. 2023 Dec;118(12):2424-2439. doi: 10.1111/add.16305. Epub 2023 Jul 29. Addiction. 2023. PMID: 37515462 Free PMC article.
-
Cost-effectiveness of new antiviral treatments for non-genotype 1 hepatitis C virus infection in China: a societal perspective.BMJ Glob Health. 2020 Nov;5(11):e003194. doi: 10.1136/bmjgh-2020-003194. BMJ Glob Health. 2020. PMID: 33246983 Free PMC article.
References
-
- Ministry of Health and Social Affairs: Statens bidrag till landstingen för kostnader för läkemedelsförmånerna m.m. för år 2015 [government transfers to the county council for costs linked to the pharmaceutical benefit}. Stockholm (2015)
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

