Tonic Serotonin Measurements In Vivo Using N-Shaped Multiple Cyclic Square Wave Voltammetry
- PMID: 34855368
- PMCID: PMC9393895
- DOI: 10.1021/acs.analchem.1c02131
Tonic Serotonin Measurements In Vivo Using N-Shaped Multiple Cyclic Square Wave Voltammetry
Abstract
Here, we present the development of a novel voltammetric technique, N-shaped multiple cyclic square wave voltammetry (N-MCSWV) and its application in vivo. It allows quantitative measurements of tonic extracellular levels of serotonin in vivo with mitigated fouling effects. N-MCSWV enriches the electrochemical information by generating high dimensional voltammograms, which enables high sensitivity and selectivity against 5-hydroindoleacetic acid (5-HIAA), dopamine, 3,4-dihydroxyphenylacetic acid (DOPAC), histamine, ascorbic acid, norepinephrine, adenosine, and pH. Using N-MCSWV, in combination with PEDOT:Nafion-coated carbon fiber microelectrodes, a tonic serotonin concentration of 52 ± 5.8 nM (n = 20 rats, ±SEM) was determined in the substantia nigra pars reticulata of urethane-anesthetized rats. Pharmacological challenges with dopaminergic, noradrenergic, and serotonergic synaptic reuptake inhibitors supported the ability of N-MCSWV to selectively detect tonic serotonin levels in vivo. Overall, N-MCSWV is a novel voltammetric technique for analytical quantification of serotonin. It offers continuous monitoring of changes in tonic serotonin concentrations in the brain to further our understanding of the role of serotonin in normal behaviors and psychiatric disorders.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
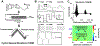


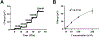


References
-
- David DJ; Gardier AM Encephale 2016, 42, 255–263. - PubMed
-
- Göthert M Pharmacol. Rep 2013, 65, 771–786. - PubMed
-
- Wilson GT; Grilo CM; Vitousek KM Am. Psychol 2007, 62, 199–216. - PubMed
-
- Hamblen JL; Norman SB; Sonis JH; Phelps AJ; Bisson JI; Nunes VD; Megnin-Viggars O; Forbes D; Riggs DS; Schnurr PP Psychotherapy 2019, 56, 359–373. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

