Randomized Phase III BMT CTN Trial of Calcineurin Inhibitor-Free Chronic Graft-Versus-Host Disease Interventions in Myeloablative Hematopoietic Cell Transplantation for Hematologic Malignancies
- PMID: 34855460
- PMCID: PMC8797487
- DOI: 10.1200/JCO.21.02293
Randomized Phase III BMT CTN Trial of Calcineurin Inhibitor-Free Chronic Graft-Versus-Host Disease Interventions in Myeloablative Hematopoietic Cell Transplantation for Hematologic Malignancies
Abstract
Purpose: Calcineurin inhibitors (CNI) are standard components of graft-versus-host disease (GVHD) prophylaxis after hematopoietic cell transplantation (HCT). Prior data suggested that CNI-free approaches using donor T-cell depletion, either by ex vivo CD34 selection or in vivo post-transplant cyclophosphamide (PTCy) as a single agent, are associated with lower rates of chronic GVHD (cGVHD).
Methods: This multicenter phase III trial randomly assigned patients with acute leukemia or myelodysplasia and an HLA-matched donor to receive CD34-selected peripheral blood stem cell, PTCy after a bone marrow (BM) graft, or tacrolimus and methotrexate after BM graft (control). The primary end point was cGVHD (moderate or severe) or relapse-free survival (CRFS).
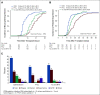
Results: Among 346 patients enrolled, 327 received HCT, 300 per protocol. Intent-to-treat rates of 2-year CRFS were 50.6% for CD34 selection (hazard ratio [HR] compared with control, 0.80; 95% CI, 0.56 to 1.15; P = .24), 48.1% for PTCy (HR, 0.86; 0.61 to 1.23; P = .41), and 41.0% for control. Corresponding rates of overall survival were 60.1% (HR, 1.74; 1.09 to 2.80; P = .02), 76.2% (HR, 1.02; 0.60 to 1.72; P = .95), and 76.1%. CD34 selection was associated with lower moderate to severe cGVHD (HR, 0.25; 0.12 to 0.52; P = .02) but higher transplant-related mortality (HR, 2.76; 1.26 to 6.06; P = .01). PTCy was associated with comparable cGVHD and survival outcomes to control, and a trend toward lower disease relapse (HR, 0.52; 0.28 to 0.96; P = .037).
Conclusion: CNI-free interventions as performed herein did not result in superior CRFS compared with tacrolimus and methotrexate with BM. Lower rates of moderate and severe cGVHD did not translate into improved survival.
Trial registration: ClinicalTrials.gov NCT02345850.
Conflict of interest statement
Figures

References
-
- Appelbaum FR: Hematopoietic-cell transplantation at 50. N Engl J Med 357:1472-1475, 2007 - PubMed
-
- Storb R, Deeg HJ, Whitehead J, et al. : Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med 314:729-735, 1986 - PubMed
-
- Bacigalupo A, Van Lint MT, Occhini D, et al. : Increased risk of leukemia relapse with high-dose cyclosporine A after allogeneic marrow transplantation for acute leukemia. Blood 77:1423-1428, 1991 - PubMed
-
- Sorror ML, Leisenring W, Deeg HJ, et al. : Twenty-year follow-up of a controlled trial comparing a combination of methotrexate plus cyclosporine with cyclosporine alone for prophylaxis of graft-versus-host disease in patients administered HLA-identical marrow grafts for leukemia. Biol Blood Marrow Transplant 11:814-815, 2005 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U10 HL069294/HL/NHLBI NIH HHS/United States
- UG1 HL109322/HL/NHLBI NIH HHS/United States
- U01 HL069310/HL/NHLBI NIH HHS/United States
- UG1 HL069249/HL/NHLBI NIH HHS/United States
- U24 HL138660/HL/NHLBI NIH HHS/United States
- UG1 HL069310/HL/NHLBI NIH HHS/United States
- UG1 HL108945/HL/NHLBI NIH HHS/United States
- UG1 HL108987/HL/NHLBI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- UG1 HL069291/HL/NHLBI NIH HHS/United States
- UG1 HL069315/HL/NHLBI NIH HHS/United States
- UG1 HL138645/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

