Pharmacokinetics and Safety of the Abacavir/Lamivudine/Lopinavir/Ritonavir Fixed-Dose Granule Formulation (4-in-1) in Neonates: PETITE Study
- PMID: 34855626
- PMCID: PMC8826609
- DOI: 10.1097/QAI.0000000000002871
Pharmacokinetics and Safety of the Abacavir/Lamivudine/Lopinavir/Ritonavir Fixed-Dose Granule Formulation (4-in-1) in Neonates: PETITE Study
Abstract
Background: Antiretroviral options for neonates (younger than 28 days) should be expanded. We evaluated the pharmacokinetics, safety, and acceptability of the "4-in-1" fixed-dose pediatric granule formulation of abacavir/lamivudine/lopinavir/ritonavir (30/15/40/10 mg) in neonates.
Methods: The PETITE study is an ongoing phase I/II, open-label, single-arm, 2-stage trial conducted in South Africa. In stage 1, term neonates exposed to HIV on standard antiretroviral prophylaxis (nevirapine ± zidovudine) received single dose(s) of the 4-in-1 formulation, followed by intensive pharmacokinetic sampling and safety assessments. At each PK visit, blood was drawn after an observed dose at 1, 2, 4, 8, and 12 hours postdose. In this study, we have reported the planned interim pharmacokinetic and safety analysis after completion of the single-dose administration.
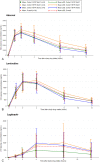
Results: Sixteen neonates, with a median (range) birth weight of 3130 g (2790-3590 g), completed 24 pharmacokinetic visits. The 4-in-1 formulation imposed relatively high doses of abacavir [8.6 mg/kg (6.6-11.4)] and lamivudine [4.3 mg/kg (3.3-5.7)] but lower doses of lopinavir [11.5 mg/kg (8.8-15.2)]. The geometric means (GM, 90% CI) AUC0-12 of abacavir, lamivudine, and lopinavir were 29.87 (26.29-33.93), 12.61 (10.72-14.83), and 3.49 (2.13-5.72) µg.h/mL, respectively. Lopinavir GM AUC0-12 was below the predefined target (20-100 µg.h/mL), and ritonavir concentrations were only detectable in 4 of the 120 (3%) samples. No adverse events were related to study drugs. No neonate had difficulty swallowing the 4-in-1 formulation.
Conclusions: The high doses of abacavir and lamivudine (in mg/kg) and AUCs were safe, and the formulation was well tolerated; however, lopinavir/ritonavir exposures were extremely low, preventing its use in neonates use in neonates. Alternative pediatric solid antiretroviral formulations must be studied in neonates.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
References
-
- Kaletra [package insert] (Product Ladeling). Abbott Park, IL: Abbott Laboratories; 2012.
-
- Penazzato M, Townsend CL, Rakhmanina N, et al. . Prioritising the most needed paediatric antiretroviral formulations: the PADO4 list. Lancet HIV. 2019;6:e623–e631. - PubMed
-
- Musiime V, Fillekes Q, Kekitiinwa A, et al. . The pharmacokinetics and acceptability of lopinavir/ritonavir minitab sprinkles, tablets, and syrups in african HIV-infected children. J Acquir Immune Defic Syndr. 2014;66:148–154. - PubMed
-
- Mwanga J, Kekitiinwa A, Musiime V, et al. . Safety, pharmacokinetics and acceptability of the abc/3tc/lpv/R granules (4-in-1) in children living with HIV (3–20 kg) in Uganda: LOLIPOP study. 2020. Presented at: International Workshop on HIV Pediatrics; November 16–17, 2020; Virtual.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical