Is Our Science Representative? A Systematic Review of Racial and Ethnic Diversity in Orthopaedic Clinical Trials from 2000 to 2020
- PMID: 34855650
- PMCID: PMC9007212
- DOI: 10.1097/CORR.0000000000002050
Is Our Science Representative? A Systematic Review of Racial and Ethnic Diversity in Orthopaedic Clinical Trials from 2000 to 2020
Abstract
Background: A lack of racial and ethnic representation in clinical trials may limit the generalizability of the orthopaedic evidence base as it applies to patients in underrepresented minority populations and perpetuate existing disparities in use, complications, or functional outcomes. Although some commentators have implied the need for mandatory race or ethnicity reporting across all orthopaedic trials, the usefulness of race or ethnic reporting likely depends on the specific topic, prior evidence of disparities, and individualized study hypotheses.
Questions/purposes: In a systematic review, we asked: (1) What proportion of orthopaedic clinical trials report race or ethnicity data, and of studies that do, how many report data regarding social covariates or genomic testing? (2) What trends and associations exist for racial and ethnic reporting among these trials between 2000 and 2020? (3) What is the racial or ethnic representation of United States trial participants compared with that reported in the United States Census?
Methods: We performed a systematic review of randomized controlled trials with human participants published in three leading general-interest orthopaedic journals that focus on clinical research: The Journal of Bone and Joint Surgery, American Volume; Clinical Orthopaedics and Related Research; and Osteoarthritis and Cartilage. We searched the PubMed and Embase databases using the following inclusion criteria: English-language studies, human studies, randomized controlled trials, publication date from 2000 to 2020, and published in Clinical Orthopaedics and Related Research; The Journal of Bone and Joint Surgery, American Volume; or Osteoarthritis and Cartilage. Primary outcome measures included whether studies reported participant race or ethnicity, other social covariates (insurance status, housing or homelessness, education and literacy, transportation, income and employment, and food security and nutrition), and genomic testing. The secondary outcome measure was the racial and ethnic categorical distribution of the trial participants included in the studies reporting race or ethnicity. From our search, 1043 randomized controlled trials with 184,643 enrolled patients met the inclusion criteria. Among these studies, 21% (223 of 1043) had a small (< 50) sample size, 56% (581 of 1043) had a medium (50 to 200) sample size, and 23% (239 of 1043) had a large (> 200) sample size. Fourteen percent (141 of 1043) were based in the Northeast United States, 9.2% (96 of 1043) were in the Midwest, 4.7% (49 of 1043) were in the West, 7.2% (75 of 1043) were in the South, and 65% (682 of 1043) were outside the United States. We calculated the overall proportion of studies meeting the inclusion criteria that reported race or ethnicity. Then among the subset of studies reporting race or ethnicity, we determined the overall rate and distribution of social covariates and genomic testing reporting. We calculated the proportion of studies reporting race or ethnicity that also reported a difference in outcome by race or ethnicity. We calculated the proportion of studies reporting race or ethnicity by each year in the study period. We also calculated the proportions and 95% CIs of individual patients in each racial or ethnic category of the studies meeting the inclusion criteria.
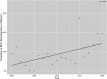
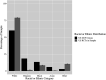
Results: During the study period (2000 to 2020), 8.5% (89 of 1043) of studies reported race or ethnicity. Of the trials reporting this factor, 4.5% (four of 89) reported insurance status, 15% (13 of 89) reported income, 4.5% (four of 89) reported housing or homelessness, 18% (16 of 89) reported education and literacy, 0% (0 of 89) reported transportation, and 2.2% (two of 89) reported food security or nutrition of trial participants. Seventy-eight percent (69 of 89) of trials reported no social covariates, while 22% (20 of 89) reported at least one. However, 0% (0 of 89) of trials reported genomic testing. Additionally, 5.6% (five of 89) of these trials reported a difference in outcomes by race or ethnicity. The proportion of studies reporting race or ethnicity increased, on average, by 0.6% annually (95% CI 0.2% to 1.0%; p = 0.02). After controlling for potentially confounding variables such as funding source, we found that studies with an increased sample size were more likely to report data by race or ethnicity; location in North America overall, Europe, Asia, and Australia or New Zealand (compared with the Northeast United States) were less likely to; and specialty-topic studies (compared with general orthopaedics research) were less likely to. Our sample of United States trials contained 18.9% more white participants than that reported in the United States Census (95% CI 18.4% to 19.4%; p < 0.001), 5.0% fewer Black participants (95% CI 4.6% to 5.3%; p < 0.001), 17.0% fewer Hispanic participants (95% CI 16.8% to 17.1%; p < 0.001), 5.3% fewer Asian participants (95% CI 5.2% to 5.4%; p < 0.001), and 7.5% more participants from other groups (95% CI 7.2% to 7.9%; p < 0.001).
Conclusion: Reporting of race or ethnicity data in orthopaedic clinical trials is low compared with other medical fields, although the proportion of diseases warranting this reporting might be lower in orthopaedics.
Clinical relevance: Investigators should initiate discussions about race and ethnicity reporting in the early stages of clinical trial development by surveying available published evidence for relevant health disparities, social determinants, and, when warranted, genomic risk factors. The decision to include or exclude race and ethnicity data in study protocols should be based on specific hypotheses, necessary statistical power, and an appreciation for unmeasured confounding. Future studies should evaluate cost-efficient mechanisms for obtaining baseline social covariate data and investigate researcher perspectives on current administrative workflows and decision-making algorithms for race and ethnicity reporting.
Copyright © 2021 by the Association of Bone and Joint Surgeons.
Conflict of interest statement
The authors certify that there are no funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article related to the author or any immediate family members. All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Figures
Comment in
-
Editor's Spotlight/Take 5: Is Our Science Representative? A Systematic Review of Racial and Ethnic Diversity in Orthopaedic Clinical Trials from 2000 to 2020.Clin Orthop Relat Res. 2022 May 1;480(5):843-847. doi: 10.1097/CORR.0000000000002193. Epub 2022 Mar 23. Clin Orthop Relat Res. 2022. PMID: 35323147 Free PMC article. No abstract available.
Similar articles
-
Reporting of Participant Race and Ethnicity in Published US Pediatric Clinical Trials From 2011 to 2020.JAMA Pediatr. 2022 May 1;176(5):e220142. doi: 10.1001/jamapediatrics.2022.0142. Epub 2022 May 2. JAMA Pediatr. 2022. PMID: 35311946 Free PMC article.
-
Are orthopedic clinical trials representative? An analysis of race and ethnicity reported in clinical trials between 2007 and 2022 : Running title: representation of clinical trials in orthopedic surgery.Arch Orthop Trauma Surg. 2024 May;144(5):1977-1987. doi: 10.1007/s00402-024-05285-7. Epub 2024 Mar 30. Arch Orthop Trauma Surg. 2024. PMID: 38554209
-
The role of race and ethnicity in the State Children's Health Insurance Program (SCHIP) in four states: are there baseline disparities, and what do they mean for SCHIP?Pediatrics. 2003 Dec;112(6 Pt 2):e521. Pediatrics. 2003. PMID: 14654674
-
United States-Based Colorectal Cancer Surgical Trials Lack Representation and Adequate Reporting of Racially and Ethnically Diverse Participants: Systematic Review and Regression Analysis.Dis Colon Rectum. 2024 May 1;67(5):624-633. doi: 10.1097/DCR.0000000000003217. Epub 2024 Jan 25. Dis Colon Rectum. 2024. PMID: 38276952
-
Racial, ethnic, and socioeconomic disparities in clinical trial reporting for metastatic spine tumors: An exploration of North American studies.Neurosurg Rev. 2025 Feb 19;48(1):247. doi: 10.1007/s10143-025-03343-1. Neurosurg Rev. 2025. PMID: 39969615 Free PMC article. Review.
Cited by
-
Underrepresentation of Non-White Participants in the American Academy of Orthopaedic Surgeons Guidelines for Surgical Management of Knee Osteoarthritis.J Arthroplasty. 2024 Feb;39(2):520-526. doi: 10.1016/j.arth.2023.08.023. Epub 2023 Aug 11. J Arthroplasty. 2024. PMID: 37572721 Free PMC article.
-
Are Orthopaedic Clinical Trials Linguistically and Culturally Diverse? A Systematic Review.JBJS Rev. 2024 May;12(5):e24.00012. doi: 10.2106/JBJS.RVW.24.00012. Epub 2024 May 3. JBJS Rev. 2024. PMID: 39021638 Free PMC article.
-
Improving Randomized-Controlled Trials in Foot and Ankle Orthopaedics: The Need to Include Sociodemographic Patient Data.Foot Ankle Spec. 2025 Aug;18(4):389-393. doi: 10.1177/19386400231170965. Epub 2023 May 6. Foot Ankle Spec. 2025. PMID: 37148174 Free PMC article. Review.
-
¿Donde están? Hispanic/Latine inclusion, diversity and representation in the HEALthy Brain and Child Development Study (HBCD).Dev Cogn Neurosci. 2024 Dec;70:101477. doi: 10.1016/j.dcn.2024.101477. Epub 2024 Nov 14. Dev Cogn Neurosci. 2024. PMID: 39561678 Free PMC article.
-
Trends and predictors of reporting social determinants of health in shoulder surgery.JSES Int. 2024 Jul 18;8(6):1259-1267. doi: 10.1016/j.jseint.2024.07.001. eCollection 2024 Nov. JSES Int. 2024. PMID: 39822844 Free PMC article.
References
-
- Alamanda VK, Song Y, Schwartz HS, Holt GE. Racial disparities in extremity soft-tissue sarcoma outcomes a nationwide analysis. Am J Clin Oncol Cancer Clin Trials. 2015;38:595-599. - PubMed
-
- Amen TB, Varady NH, Rajaee S, Chen AF. Persistent racial disparities in utilization rates and perioperative metrics in total joint arthroplasty in the U.S. J Bone Joint Surg Am . 2020;102:811-820. - PubMed
-
- Beck JJ, Pandya NK, Carter CW, Mulcahey MK. Current concept review: inclusion and analysis of diverse study populations in orthopaedic research. J Am Acad Orthop Surg . 2021;29:e479-e487. - PubMed
-
- Bhandari M, Richards RR, Sprague S, Schemitsch EH. The quality of reporting of randomized trials in the Journal of Bone and Joint Surgery from 1988 through 2000. J Bone Joint Surg Am. 2002;84:388-396. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

