A translational rat model for ex vivo lung perfusion of pre-injured lungs after brain death
- PMID: 34855870
- PMCID: PMC8638921
- DOI: 10.1371/journal.pone.0260705
A translational rat model for ex vivo lung perfusion of pre-injured lungs after brain death
Abstract
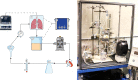
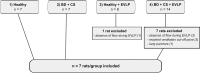
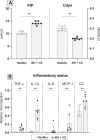
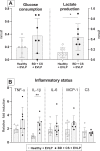
The process of brain death (BD) detrimentally affects donor lung quality. Ex vivo lung perfusion (EVLP) is a technique originally designed to evaluate marginal donor lungs. Nowadays, its potential as a treatment platform to repair damaged donor lungs is increasingly studied in experimental models. Rat models for EVLP have been described in literature before, yet the pathophysiology of BD was not included in these protocols and prolonged perfusion over 3 hours without anti-inflammatory additives was not achieved. We aimed to establish a model for prolonged EVLP of rat lungs from brain-dead donors, to provide a reliable platform for future experimental studies. Rat lungs were randomly assigned to one of four experimental groups (n = 7/group): 1) healthy, directly procured lungs, 2) lungs procured from rats subjected to 3 hours of BD and 1 hour cold storage (CS), 3) healthy, directly procured lungs subjected to 6 hours EVLP and 4), lungs procured from rats subjected to 3 hours of BD, 1 hour CS and 6 hours EVLP. Lungs from brain-dead rats showed deteriorated ventilation parameters and augmented lung damage when compared to healthy controls, in accordance with the pathophysiology of BD. Subsequent ex vivo perfusion for 6 hours was achieved, both for lungs of healthy donor rats as for pre-injured donor lungs from brain-dead rats. The worsened quality of lungs from brain-dead donors was evident during EVLP as well, as corroborated by deteriorated ventilation performance, increased lactate production and augmented inflammatory status during EVLP. In conclusion, we established a stable model for prolonged EVLP of pre-injured lungs from brain-dead donor rats. In this report we describe tips and pitfalls in the establishment of the rat EVLP model, to enhance reproducibility by other researchers.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Cypel M, Yeung JC, Donahoe L, et al. Normothermic ex vivo lung perfusion: Does the indication impact organ utilization and patient outcomes after transplantation? J Thorac Cardiovasc Surg 2020; 159: 346–355.e1. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

