Prospective comparison of CT and 18F-FDG PET/MRI in N and M staging of primary breast cancer patients: Initial results
- PMID: 34855886
- PMCID: PMC8638872
- DOI: 10.1371/journal.pone.0260804
Prospective comparison of CT and 18F-FDG PET/MRI in N and M staging of primary breast cancer patients: Initial results
Abstract
Objectives: To compare the diagnostic accuracy of contrast-enhanced thoraco-abdominal computed tomography and whole-body 18F-FDG PET/MRI in N and M staging in newly diagnosed, histopathological proven breast cancer.
Material and methods: A total of 80 consecutive women with newly diagnosed and histopathologically confirmed breast cancer were enrolled in this prospective study. Following inclusion criteria had to be fulfilled: (1) newly diagnosed, treatment-naive T2-tumor or higher T-stage or (2) newly diagnosed, treatment-naive triple-negative tumor of every size or (3) newly diagnosed, treatment-naive tumor with molecular high risk (T1c, Ki67 >14%, HER2neu over-expression, G3). All patients underwent a thoraco-abdominal ceCT and a whole-body 18F-FDG PET/MRI. All datasets were evaluated by two experienced radiologists in hybrid imaging regarding suspect lesion count, localization, categorization and diagnostic confidence. Images were interpreted in random order with a reading gap of at least 4 weeks to avoid recognition bias. Histopathological results as well as follow-up imaging served as reference standard. Differences in staging accuracy were assessed using Mc Nemars chi2 test.
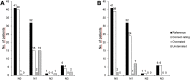
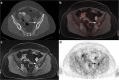
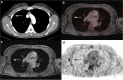
Results: CT rated the N stage correctly in 64 of 80 (80%, 95% CI:70.0-87.3) patients with a sensitivity of 61.5% (CI:45.9-75.1), a specificity of 97.6% (CI:87.4-99.6), a PPV of 96% (CI:80.5-99.3), and a NPV of 72.7% (CI:59.8-82.7). Compared to this, 18F-FDG PET/MRI determined the N stage correctly in 71 of 80 (88.75%, CI:80.0-94.0) patients with a sensitivity of 82.1% (CI:67.3-91.0), a specificity of 95.1% (CI:83.9-98.7), a PPV of 94.1% (CI:80.9-98.4) and a NPV of 84.8% (CI:71.8-92.4). Differences in sensitivities were statistically significant (difference 20.6%, CI:-0.02-40.9; p = 0.008). Distant metastases were present in 7/80 patients (8.75%). 18 F-FDG PET/MRI detected all of the histopathological proven metastases without any false-positive findings, while 3 patients with bone metastases were missed in CT (sensitivity 57.1%, specificity 95.9%). Additionally, CT presented false-positive findings in 3 patients.
Conclusion: 18F-FDG PET/MRI has a high diagnostic potential and outperforms CT in assessing the N and M stage in patients with primary breast cancer.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- The Global Cancer Observatory G. Breast Cancer. Source: Globocan 2018. World Heal Organ. 2018;876: 2018–2019.
-
- Wockel A, Festl J, Stuber T, Brust K, Krockenberger M, Heuschmann PU, et al.. Interdisciplinary Screening, Diagnosis, Therapy and Follow-up of Breast Cancer. Guideline of the DGGG and the DKG (S3-Level, AWMF Registry Number 032/045OL, December 2017)—Part 2 with Recommendations for the Therapy of Primary, Recurrent and Advanced Br. Geburtshilfe Frauenheilkd. 2018;78: 1056–1088. doi: 10.1055/a-0646-4630 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

