Niclosamide shows strong antiviral activity in a human airway model of SARS-CoV-2 infection and a conserved potency against the Alpha (B.1.1.7), Beta (B.1.351) and Delta variant (B.1.617.2)
- PMID: 34855904
- PMCID: PMC8639074
- DOI: 10.1371/journal.pone.0260958
Niclosamide shows strong antiviral activity in a human airway model of SARS-CoV-2 infection and a conserved potency against the Alpha (B.1.1.7), Beta (B.1.351) and Delta variant (B.1.617.2)
Abstract
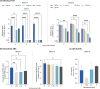
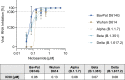
SARS-CoV-2 variants are emerging with potential increased transmissibility highlighting the great unmet medical need for new therapies. Niclosamide is a potent anti-SARS-CoV-2 agent that has advanced in clinical development. We validate the potent antiviral efficacy of niclosamide in a SARS-CoV-2 human airway model. Furthermore, niclosamide remains its potency against the D614G, Alpha (B.1.1.7), Beta (B.1.351), and Delta (B.1.617.2) variants. Our data further support the potent anti-SARS-CoV-2 properties of niclosamide and highlights its great potential as a therapeutic agent for COVID-19.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: MOAS and AW are shareholders or benefit from an employee incentive scheme in UNION therapeutics. This does not alter our adherence to PLOS ONE policies on sharing data and materials. Further, UNION had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All other authors have nothing to disclose.
Figures
Similar articles
-
Efficacy of Niclosamide vs Placebo in SARS-CoV-2 Respiratory Viral Clearance, Viral Shedding, and Duration of Symptoms Among Patients With Mild to Moderate COVID-19: A Phase 2 Randomized Clinical Trial.JAMA Netw Open. 2022 Feb 1;5(2):e2144942. doi: 10.1001/jamanetworkopen.2021.44942. JAMA Netw Open. 2022. PMID: 35138402 Free PMC article. Clinical Trial.
-
Inhalable spray-dried dry powders combining ivermectin and niclosamide to inhibit SARS-CoV-2 infection in vitro.Int J Pharm. 2025 Feb 25;671:125302. doi: 10.1016/j.ijpharm.2025.125302. Epub 2025 Jan 30. Int J Pharm. 2025. PMID: 39892677
-
Curcumin Inhibits In Vitro SARS-CoV-2 Infection In Vero E6 Cells through Multiple Antiviral Mechanisms.Molecules. 2021 Nov 16;26(22):6900. doi: 10.3390/molecules26226900. Molecules. 2021. PMID: 34833991 Free PMC article.
-
Niclosamide for Covid-19: bridging the gap.Mol Biol Rep. 2021 Dec;48(12):8195-8202. doi: 10.1007/s11033-021-06770-7. Epub 2021 Oct 18. Mol Biol Rep. 2021. PMID: 34664162 Free PMC article. Review.
-
Niclosamide-A promising treatment for COVID-19.Br J Pharmacol. 2022 Jul;179(13):3250-3267. doi: 10.1111/bph.15843. Epub 2022 Apr 11. Br J Pharmacol. 2022. PMID: 35348204 Free PMC article. Review.
Cited by
-
The Endolysosomal System: The Acid Test for SARS-CoV-2.Int J Mol Sci. 2022 Apr 20;23(9):4576. doi: 10.3390/ijms23094576. Int J Mol Sci. 2022. PMID: 35562967 Free PMC article. Review.
-
In vitro activity of therapeutic antibodies against SARS-CoV-2 Omicron BA.1, BA.2 and BA.5.Sci Rep. 2022 Jul 23;12(1):12609. doi: 10.1038/s41598-022-16964-z. Sci Rep. 2022. PMID: 35871089 Free PMC article.
-
An Ensemble Matrix Completion Model for Predicting Potential Drugs Against SARS-CoV-2.Front Microbiol. 2021 Jul 21;12:694534. doi: 10.3389/fmicb.2021.694534. eCollection 2021. Front Microbiol. 2021. PMID: 34367094 Free PMC article.
-
Prophylaxis for renal patients at risk of COVID-19 infection: results from the intranasal niclosamide randomised, double blinded, placebo controlled arm of the PROTECT-V platform trial.BMC Infect Dis. 2025 Feb 11;25(1):204. doi: 10.1186/s12879-025-10584-4. BMC Infect Dis. 2025. PMID: 39934669 Free PMC article. Clinical Trial.
-
Identification of niclosamide as a novel antiviral agent against porcine epidemic diarrhea virus infection by targeting viral internalization.Virol Sin. 2023 Apr;38(2):296-308. doi: 10.1016/j.virs.2023.01.008. Epub 2023 Jan 23. Virol Sin. 2023. PMID: 36702255 Free PMC article.
References
-
- Johnson & Johnson Announces Single-Shot Janssen COVID-19 Vaccine Candidate Met Primary Endpoints in Interim Analysis of its Phase 3 ENSEMBLE Trial | Janssen. [cited 26 Apr 2021]. Available: https://www.janssen.com/johnson-johnson-announces-single-shot-janssen-co...
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Miscellaneous

