Association of clonal hematopoiesis with chronic obstructive pulmonary disease
- PMID: 34855941
- PMCID: PMC8777202
- DOI: 10.1182/blood.2021013531
Association of clonal hematopoiesis with chronic obstructive pulmonary disease
Erratum in
-
Miller PG, Qiao D, Rojas-Quintero J, et al. Association of clonal hematopoiesis with chronic obstructive pulmonary disease. Blood. 2022;139(3):357-368.Blood. 2023 Feb 9;141(6):682. doi: 10.1182/blood.2022018840. Blood. 2023. PMID: 36757726 Free PMC article. No abstract available.
Abstract
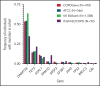
Chronic obstructive pulmonary disease (COPD) is associated with age and smoking, but other determinants of the disease are incompletely understood. Clonal hematopoiesis of indeterminate potential (CHIP) is a common, age-related state in which somatic mutations in clonal blood populations induce aberrant inflammatory responses. Patients with CHIP have an elevated risk for cardiovascular disease, but the association of CHIP with COPD remains unclear. We analyzed whole-genome sequencing and whole-exome sequencing data to detect CHIP in 48 835 patients, of whom 8444 had moderate to very severe COPD, from four separate cohorts with COPD phenotyping and smoking history. We measured emphysema in murine models in which Tet2 was deleted in hematopoietic cells. In the COPDGene cohort, individuals with CHIP had risks of moderate-to-severe, severe, or very severe COPD that were 1.6 (adjusted 95% confidence interval [CI], 1.1-2.2) and 2.2 (adjusted 95% CI, 1.5-3.2) times greater than those for noncarriers. These findings were consistently observed in three additional cohorts and meta-analyses of all patients. CHIP was also associated with decreased FEV1% predicted in the COPDGene cohort (mean between-group differences, -5.7%; adjusted 95% CI, -8.8% to -2.6%), a finding replicated in additional cohorts. Smoke exposure was associated with a small but significant increased risk of having CHIP (odds ratio, 1.03 per 10 pack-years; 95% CI, 1.01-1.05 per 10 pack-years) in the meta-analysis of all patients. Inactivation of Tet2 in mouse hematopoietic cells exacerbated the development of emphysema and inflammation in models of cigarette smoke exposure. Somatic mutations in blood cells are associated with the development and severity of COPD, independent of age and cumulative smoke exposure.
© 2022 by The American Society of Hematology.
Figures



Comment in
-
COPD makes CHIP less indeterminate.Blood. 2022 Jan 20;139(3):310-311. doi: 10.1182/blood.2021013966. Blood. 2022. PMID: 35050339 No abstract available.
References
-
- Ford ES, Mannino DM, Wheaton AG, Giles WH, Presley-Cantrell L, Croft JB. Trends in the prevalence of obstructive and restrictive lung function among adults in the United States: findings from the National Health and Nutrition Examination surveys from 1988-1994 to 2007-2010. Chest. 2013;143(5):1395-1406. - PMC - PubMed
-
- Kochanek KD, Murphy S, Xu J, Arias E. Mortality in the United States, 2016. NCHS Data Brief. 2017;(293):1-8. - PubMed
-
- Hubeau C, Kubera JE, Masek-Hammerman K, Williams CM. Interleukin-6 neutralization alleviates pulmonary inflammation in mice exposed to cigarette smoke and poly(I:C). Clin Sci (Lond). 2013;125(10):483-493. - PubMed
-
- Grubek-Jaworska H, Paplińska M, Hermanowicz-Salamon J, et al. IL-6 and IL-13 in induced sputum of COPD and asthma patients: correlation with respiratory tests. Respiration. 2012;84(2):101-107. - PubMed
Publication types
MeSH terms
Grants and funding
- U54 HG003067/HG/NHGRI NIH HHS/United States
- R01 HL071259/HL/NHLBI NIH HHS/United States
- R01 HL092577/HL/NHLBI NIH HHS/United States
- 75N92020D00001/HL/NHLBI NIH HHS/United States
- N01 HC095167/HL/NHLBI NIH HHS/United States
- K12 CA087723/CA/NCI NIH HHS/United States
- R01 HL071205/HL/NHLBI NIH HHS/United States
- N01 HC095166/HL/NHLBI NIH HHS/United States
- R01 HL113264/HL/NHLBI NIH HHS/United States
- T32 HL094301/HL/NHLBI NIH HHS/United States
- UL1 RR033176/RR/NCRR NIH HHS/United States
- 75N92020D00002/HL/NHLBI NIH HHS/United States
- U54 HG006493/HG/NHGRI NIH HHS/United States
- R35 CA253125/CA/NCI NIH HHS/United States
- N01 HC095161/HL/NHLBI NIH HHS/United States
- 75N92020D00005/HL/NHLBI NIH HHS/United States
- N01 HC095168/HL/NHLBI NIH HHS/United States
- R01 HL071251/HL/NHLBI NIH HHS/United States
- K08 CA252174/CA/NCI NIH HHS/United States
- R01 HL120393/HL/NHLBI NIH HHS/United States
- UL1 TR001079/TR/NCATS NIH HHS/United States
- N01 HC095169/HL/NHLBI NIH HHS/United States
- U01 HL089897/HL/NHLBI NIH HHS/United States
- R01 HL077612/HL/NHLBI NIH HHS/United States
- R01 HL153805/HL/NHLBI NIH HHS/United States
- U01 HL120393/HL/NHLBI NIH HHS/United States
- R01 HL082945/HL/NHLBI NIH HHS/United States
- R01 HL071250/HL/NHLBI NIH HHS/United States
- R01 HL086694/HL/NHLBI NIH HHS/United States
- U01 HG004402/HG/NHGRI NIH HHS/United States
- R01 HL089856/HL/NHLBI NIH HHS/United States
- U54 HG003273/HG/NHGRI NIH HHS/United States
- 75N92020D00003/HL/NHLBI NIH HHS/United States
- K08 CA248960/CA/NCI NIH HHS/United States
- N01 HC095159/HL/NHLBI NIH HHS/United States
- P01 CA066996/CA/NCI NIH HHS/United States
- U01 HL089856/HL/NHLBI NIH HHS/United States
- R01 HL135142/HL/NHLBI NIH HHS/United States
- R35 HL135818/HL/NHLBI NIH HHS/United States
- P30 DK063491/DK/NIDDK NIH HHS/United States
- R01 HL071051/HL/NHLBI NIH HHS/United States
- N01 HC025195/HL/NHLBI NIH HHS/United States
- P01 CA108631/CA/NCI NIH HHS/United States
- R01 HL068111/HL/NHLBI NIH HHS/United States
- UL1 TR001420/TR/NCATS NIH HHS/United States
- 75N92020D00004/HL/NHLBI NIH HHS/United States
- DP2 HL157540/HL/NHLBI NIH HHS/United States
- N01 HC095163/HL/NHLBI NIH HHS/United States
- 75N92020D00007/HL/NHLBI NIH HHS/United States
- K01 HL129039/HL/NHLBI NIH HHS/United States
- Z01 ES043012/ImNIH/Intramural NIH HHS/United States
- 75N92019D00031/HL/NHLBI NIH HHS/United States
- R03 HL154284/HL/NHLBI NIH HHS/United States
- R01 HL098031/HL/NHLBI NIH HHS/United States
- UL1 TR000040/TR/NCATS NIH HHS/United States
- 75N92020D00006/HL/NHLBI NIH HHS/United States
- K08 CA263183/CA/NCI NIH HHS/United States
- R01 HL087641/HL/NHLBI NIH HHS/United States
- R01 HL117626/HL/NHLBI NIH HHS/United States
- N01 HC095162/HL/NHLBI NIH HHS/United States
- UL1 TR001881/TR/NCATS NIH HHS/United States
- N01 HC095165/HL/NHLBI NIH HHS/United States
- N01 HC095164/HL/NHLBI NIH HHS/United States
- R01 HL071258/HL/NHLBI NIH HHS/United States
- R01 HL148050/HL/NHLBI NIH HHS/United States
- T32 HL007427/HL/NHLBI NIH HHS/United States
- K01 HL135405/HL/NHLBI NIH HHS/United States
- N01 HC095160/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

