Exploring the COVID-19 vaccine candidates against SARS-CoV-2 and its variants: where do we stand and where do we go?
- PMID: 34856868
- PMCID: PMC8726002
- DOI: 10.1080/21645515.2021.1995283
Exploring the COVID-19 vaccine candidates against SARS-CoV-2 and its variants: where do we stand and where do we go?
Abstract
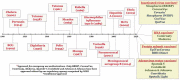
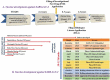
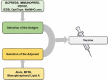
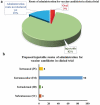
As of September 2021, 117 COVID-19 vaccines are in clinical development, and 194 are in preclinical development as per the World Health Organization (WHO) published draft landscape. Among the 117 vaccines undergoing clinical trials, the major platforms include protein subunit; RNA; inactivated virus; viral vector, among others. So far, USFDA recognized to approve the Pfizer-BioNTech (Comirnaty) COVID-19 vaccine for its full use in individuals of 16 years of age and older. Though the approved vaccines are being manufactured at a tremendous pace, the wealthiest countries have about 28% of total vaccines despite possessing only 10.8% of the total world population, suggesting an inequity of vaccine distribution. The review comprehensively summarizes the history of vaccines, mainly focusing on vaccines for SARS-CoV-2. The review also connects relevant topics, including measurement of vaccines efficacy against SARS-CoV-2 and its variants, associated challenges, and limitations, as hurdles in global vaccination are also kept forth.
Keywords: COVID-19; SARS-CoV-2; design; distribution; efficacy; equity; production; vaccines; variants.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
