Identification and Quantification of MIDD0301 Metabolites
- PMID: 34856893
- PMCID: PMC8760168
- DOI: 10.2174/1389200222666211202093841
Identification and Quantification of MIDD0301 Metabolites
Abstract
Background: MIDD0301 is an oral asthma drug candidate that binds GABAA receptors on airway smooth muscle and immune cells.
Objective: The objective of this study is to identify and quantify MIDD0301 metabolites in vitro and in vivo and determine the pharmacokinetics of oral, IP, and IV administered MIDD0301.
Methods: In vitro conversion of MIDD0301 was performed using liver and kidney microsomes/S9 fractions followed by quantification using liquid chromatography-tandem mass spectrometry (LC-MS/MS). A LC-MS/MS method was developed using synthesized standards to quantify MIDD0301 and its metabolites in urine and feces. Blood, lung, and brain were harvested from animals that received MIDD0301 by oral, IP, and IV administration, followed by LCMS/ MS quantification. Imaging mass spectrometry was used to demonstrate the presence of MIDD0301 in the lung after oral administration.
Results: MIDD0301 is stable in the presence of liver and kidney microsomes and S9 fractions for at least two hours. MIDD0301 undergoes conversion to the corresponding glucuronide and glucoside in the presence of conjugating cofactors. For IP and IV administration, unconjugated MIDD0301 together with significant amounts of MIDD0301 glucoside and MIDD0301 taurine were found in urine and feces. Less conjugation was observed following oral administration, with MIDD0301 glucuronide being the main metabolite. Pharmacokinetic quantification of MIDD0301 in blood, lung, and brain showed very low levels of MIDD0301 in the brain after oral, IV, or IP administration. The drug half-life in these tissues ranged between 4-6 hours for IP and oral and 1-2 hours for IV administration. Imaging mass spectrometry demonstrated that orally administered MIDD0301 distributes uniformly in the lung parenchyma.
Conclusion: MIDD0301 undergoes no phase I and moderate phase II metabolism.
Keywords: GABAA receptor.; MIDD0301; glucosidation; glucuronidation; imaging mass spectrometry; metabolism; taurine conjugate.
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Figures



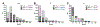

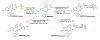
References
-
- Safety Testing of Drug Metabolites Guidance for Industry. U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) 2016.
-
- Meunier B; de Visser SP; Shaik S, Mechanism of oxidation reactions catalyzed by cytochrome p450 enzymes. Chem Rev 2004, 104 (9), 3947–80. - PubMed
-
- Vasiliou V; Pappa A; Estey T, Role of human aldehyde dehydrogenases in endobiotic and xenobiotic metabolism. Drug Metab Rev 2004, 36 (2), 279–99. - PubMed
-
- Tafazoli S; O’Brien PJ, Peroxidases: a role in the metabolism and side effects of drugs. Drug Discov Today 2005, 10 (9), 617–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

