Neurosteroid Modulation of GABAA Receptor Function by Independent Action at Multiple Specific Binding Sites
- PMID: 34856904
- PMCID: PMC9881108
- DOI: 10.2174/1570159X19666211202150041
Neurosteroid Modulation of GABAA Receptor Function by Independent Action at Multiple Specific Binding Sites
Abstract
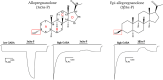
Neurosteroids are endogenous modulators of GABAA receptors that mediate anxiety, pain, mood and arousal. The 3-hydroxyl epimers, allopregnanolone (3α-OH) and epiallopregnanolone (3β-OH) are both prevalent in the mammalian brain and produce opposite effects on GABAA receptor function, acting as positive and negative allosteric modulators, respectively. This Perspective provides a model to explain the actions of 3α-OH and 3β-OH neurosteroids. The model is based on evidence that the neurosteroid epimers bind to an overlapping subset of specific sites on GABAA receptors, with their net functional effect on channel gating being the sum of their independent effects at each site.
Keywords: GABAA receptors; Neurosteroids; affinity labeling; desensitization; ion channels; structural biology.
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Figures
Similar articles
-
Site-specific effects of neurosteroids on GABAA receptor activation and desensitization.Elife. 2020 Sep 21;9:e55331. doi: 10.7554/eLife.55331. Elife. 2020. PMID: 32955433 Free PMC article.
-
Direct measurements of neurosteroid binding to specific sites on GABAA receptors.Br J Pharmacol. 2024 Nov;181(21):4229-4244. doi: 10.1111/bph.16490. Epub 2024 Jul 8. Br J Pharmacol. 2024. PMID: 38978389 Free PMC article.
-
Structural insights into opposing actions of neurosteroids on GABAA receptors.Nat Commun. 2023 Aug 22;14(1):5091. doi: 10.1038/s41467-023-40800-1. Nat Commun. 2023. PMID: 37607940 Free PMC article.
-
GABAA receptor modulating steroid antagonists (GAMSA) are functional in vivo.J Steroid Biochem Mol Biol. 2016 Jun;160:98-105. doi: 10.1016/j.jsbmb.2015.10.019. Epub 2015 Oct 30. J Steroid Biochem Mol Biol. 2016. PMID: 26523675 Review.
-
Neurosteroids and translocator protein 18 kDa (TSPO) in depression: implications for synaptic plasticity, cognition, and treatment options.Eur Arch Psychiatry Clin Neurosci. 2023 Oct;273(7):1477-1487. doi: 10.1007/s00406-022-01532-3. Epub 2022 Dec 27. Eur Arch Psychiatry Clin Neurosci. 2023. PMID: 36574032 Review.
Cited by
-
Interaction Between Allopregnanolone and Amiloride Binding Sites on the GABAA Receptor.Cell Biochem Biophys. 2025 Jun;83(2):2453-2459. doi: 10.1007/s12013-024-01654-6. Epub 2024 Dec 28. Cell Biochem Biophys. 2025. PMID: 39730891
-
Progesterone- and deoxycorticosterone-derived GABAergic neurosteroids induce itch-related scratching behavior in mouse model of atopic dermatitis.Naunyn Schmiedebergs Arch Pharmacol. 2025 Jun 2. doi: 10.1007/s00210-025-04306-5. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40455235
-
Stereospecific Properties and Intracellular Transport of Novel Intrinsically Fluorescent Neurosteroids.ACS Chem Neurosci. 2024 Dec 4;15(23):4322-4336. doi: 10.1021/acschemneuro.4c00571. Epub 2024 Nov 22. ACS Chem Neurosci. 2024. PMID: 39574303
-
Synthesis and evaluation of photoaffinity labeling reagents for identifying binding sites of sulfated neurosteroids on NMDA and GABAA receptors.RSC Adv. 2024 Nov 13;14(49):36352-36369. doi: 10.1039/d4ra07074g. eCollection 2024 Nov 11. RSC Adv. 2024. PMID: 39539530 Free PMC article.
-
The Mechanism of Enantioselective Neurosteroid Actions on GABAA Receptors.Biomolecules. 2023 Feb 9;13(2):341. doi: 10.3390/biom13020341. Biomolecules. 2023. PMID: 36830708 Free PMC article.
References
-
- Wang M., He Y., Eisenman L.N., Fields C., Zeng C.M., Mathews J., Benz A., Fu T., Zorumski E., Steinbach J.H., Covey D.F., Zorumski C.F., Mennerick S. 3beta-hydroxypregnane steroids are pregnenolone sulfate-like GABA(A) receptor antagonists. J. Neurosci. 2002;22(9):3366–3375. doi: 10.1523/JNEUROSCI.22-09-03366.2002. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

