TANGO: effect of tango Argentino on cancer-associated fatigue in breast cancer patients-study protocol for a randomized controlled trial
- PMID: 34857031
- PMCID: PMC8637025
- DOI: 10.1186/s13063-021-05869-3
TANGO: effect of tango Argentino on cancer-associated fatigue in breast cancer patients-study protocol for a randomized controlled trial
Abstract
Background: The majority of breast cancer patients suffer from persistent impairments after completion of their primary oncological therapy. Cancer-related fatigue (CRF) in particular is a multidimensional syndrome having a profound negative impact on the quality of life. To counter CRF symptoms, physical activities are suggested as first-line interventions, mind-body therapies have been shown to be effective, and music therapy can also reduce anxiety and stress in breast cancer patients. Tango therapy that combines various elements can have an impact on physical, psychological, and cognitive abilities and could therefore have a beneficial effect on breast cancer patients. The purpose of this study is to investigate whether a 6-week tango module is suited as a therapeutic approach for people after primary breast cancer therapy to favorably influence their quality of life, especially CRF levels.
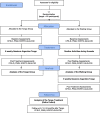
Methods: Sixty patients with a diagnosis for stage I-III breast cancer 12-48 months before enrollment and with CRF (age > 18) will be recruited and randomized 1:1 to a tango or a waiting-list group. Movement concepts using elements of Argentine tango (self-awareness, musical and spatial perception, self-perception, playfulness, shared experience) will be examined with the participants during six consecutive weekly 1-h tango sessions. The primary outcome will be the improvement of CRF (German version of the Cancer Fatigue Scale), and the secondary outcomes will be the improvement in sleep quality (Pittsburgh Sleep Quality Index) and quality of life (EORTC-QLQ-C30). Patient-reported outcomes will be measured at baseline and 6 weeks later; follow-up will be performed 6, 12, and 24 months after baseline. An evaluation will be performed by means of descriptive data analyses.
Discussion: Argentine tango, as a music-based movement therapy, can influence different skills and may improve several outcomes. The therapeutic use of Argentine tango in the care of breast cancer patients has not yet been reported. It is anticipated that participants receiving the tango module will have improved CRF, sleep, and quality of life scores compared to a waitlist control.
Trial registration: German Clinical Trials Registry (DRKS) DRKS00021601 . Retrospectively registered on 21 August 2020.
Keywords: Breast cancer; Dance; Fatigue; Health-related quality of life; Insomnia.
© 2021. The Author(s).
Conflict of interest statement
JG reports grants from Roche (honoraria for speaking). HM is a member of the board of directors of Weleda AG, a member of the Network Arbeitsgemeinschaft der Wissenschaftlichen Fachgesellschaften (AWMF e.V.) guideline committee for integrative oncology (Guideline for Complementary Medicine in the Treatment of Oncological Patients), and has an endowed professorship at the Charité Universitätsmedizin Berlin, which is financed by the Software AG Foundation, outside the submitted work. FS reports grants from Helixor Heilmittel GmbH (travel costs and honoraria for speaking), grants from AstraZeneca (travel costs and honoraria for speaking), grants from Abnoba GmbH, and grants from Iscador AG, outside the submitted work. The other authors declared that they have no competing interests. No payment was received for any other aspects of the submitted work. There are no patents, products in the development, or marketed products to declare. There are no other relationships/conditions/circumstances that present a potential conflict of interest.
Figures
Similar articles
-
Does the Argentine Tango Sustainably Improve Cancer-Associated Fatigue and Quality of Life in Breast Cancer Survivors?Cancers (Basel). 2023 Nov 30;15(23):5678. doi: 10.3390/cancers15235678. Cancers (Basel). 2023. PMID: 38067381 Free PMC article.
-
Efficacy of Tango Argentino for Cancer-Associated Fatigue and Quality of Life in Breast Cancer Survivors: A Randomized Controlled Trial.Cancers (Basel). 2023 May 26;15(11):2920. doi: 10.3390/cancers15112920. Cancers (Basel). 2023. PMID: 37296883 Free PMC article.
-
Light-enhanced cognitive behavioural therapy for sleep and fatigue: study protocol for a randomised controlled trial during chemotherapy for breast cancer.Trials. 2020 Mar 27;21(1):295. doi: 10.1186/s13063-020-4196-4. Trials. 2020. PMID: 32216832 Free PMC article.
-
Telephone interventions for symptom management in adults with cancer.Cochrane Database Syst Rev. 2020 Jun 2;6(6):CD007568. doi: 10.1002/14651858.CD007568.pub2. Cochrane Database Syst Rev. 2020. PMID: 32483832 Free PMC article.
-
Effects of exercise interventions on cancer-related fatigue in breast cancer patients: an overview of systematic reviews.Support Care Cancer. 2022 Dec;30(12):10421-10440. doi: 10.1007/s00520-022-07389-5. Epub 2022 Nov 3. Support Care Cancer. 2022. PMID: 36326908 Free PMC article. Review.
Cited by
-
Does the Argentine Tango Sustainably Improve Cancer-Associated Fatigue and Quality of Life in Breast Cancer Survivors?Cancers (Basel). 2023 Nov 30;15(23):5678. doi: 10.3390/cancers15235678. Cancers (Basel). 2023. PMID: 38067381 Free PMC article.
-
Efficacy of thunder-fire moxibustion for cancer-related fatigue in breast cancer survivors: study protocol for a randomized controlled trial.Front Oncol. 2025 Mar 11;15:1496741. doi: 10.3389/fonc.2025.1496741. eCollection 2025. Front Oncol. 2025. PMID: 40134594 Free PMC article.
-
Efficacy of Tango Argentino for Cancer-Associated Fatigue and Quality of Life in Breast Cancer Survivors: A Randomized Controlled Trial.Cancers (Basel). 2023 May 26;15(11):2920. doi: 10.3390/cancers15112920. Cancers (Basel). 2023. PMID: 37296883 Free PMC article.
-
Effect of Dance Movement Therapy on Cancer-Related Fatigue in Breast Cancer Patients Undergoing Radiation Therapy: A Pre-post Intervention Study.Cureus. 2022 Jan 9;14(1):e21040. doi: 10.7759/cureus.21040. eCollection 2022 Jan. Cureus. 2022. PMID: 35155008 Free PMC article.
References
-
- Cella D, Peterman A, Passik S, Jacobsen P, Breitbart W. Progress toward guidelines for the management of fatigue. Oncology (Williston Park, NY). 1998;12(11A):369-77. PubMed PMID: 10028520. Epub 1999/02/24. eng. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous