Burden of cystic fibrosis in children <12 years of age prior to the introduction of CFTR modulator therapies
- PMID: 34857524
- PMCID: PMC8640656
- DOI: 10.1136/bmjresp-2021-000998
Burden of cystic fibrosis in children <12 years of age prior to the introduction of CFTR modulator therapies
Abstract
Background: Cystic fibrosis (CF) is a genetic, multisystemic, progressive and life-shortening disease caused by mutations in the CF transmembrane conductance regulator (CFTR) gene. Different genotypes have been linked to variations in disease progression among people with CF. The burden of illness (BOI) in children with CF is incompletely characterised, particularly as it relates to CFTR genotypes prior to the availability of the first CFTR modulators. This retrospective, cross-sectional, descriptive study evaluated the BOI in US children with CF <12 years of age prior to the first approval of CFTR modulators.
Methods: Data from the US Cystic Fibrosis Foundation Patient Registry from 2011 were used to summarise key patient and disease characteristics using descriptive statistics, overall and grouped by age (0 to <2 years, 2 to <6 years and 6 to <12 years) and genotype (F508del/F508del, F508del/minimal function (MF), MF/MF, gating mutation on ≥1 allele, residual function mutation on ≥1 allele and R117H on ≥1 allele) group.
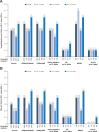
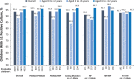
Results: The analysis included 9185 children. Among 6-year-olds to <12-year-olds, mean (SD) per cent predicted FEV1 in 1 s was 92.6% (17.5%). Among all children <12 years of age, the mean (SD) all-cause hospitalisation and pulmonary exacerbation rates in 2011 were 0.4 (1.0) and 0.3 (0.8), respectively. Most (93.6%) had ≥1 positive lung microbiology culture. CF-related medication and nutritional supplementation use was common across all ages and genotypes. More than half (54.7%) had ≥1 CF-related complication. Evidence of disease burden was observed across the age and genotype groups studied.
Conclusions: Prior to the approval of the first CFTR modulator therapies in children <12 years of age, CF was associated with substantial BOI from an early age-including respiratory infections, hospitalisations/pulmonary exacerbations, need for supplemental nutrition and pharmacological treatments-irrespective of genotype.
Keywords: cystic fibrosis.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: KB, EA-S, GL and CLC are employees of Vertex Pharmaceuticals Incorporated and may own stock or stock options in that company. SJM is an employee of ICON Clinical Research, which received funding from Vertex Pharmaceuticals Incorporated for analytic consulting services for this study and which receives funding from various pharmaceutical, biotechnology and device companies for providing clinical research services.
Figures

Similar articles
-
Disease burden in people with cystic fibrosis heterozygous for F508del and a minimal function mutation.J Cyst Fibros. 2022 Jan;21(1):96-103. doi: 10.1016/j.jcf.2021.07.003. Epub 2021 Jul 19. J Cyst Fibros. 2022. PMID: 34289939 Free PMC article.
-
Cystic Fibrosis Foundation Pulmonary Guidelines. Use of Cystic Fibrosis Transmembrane Conductance Regulator Modulator Therapy in Patients with Cystic Fibrosis.Ann Am Thorac Soc. 2018 Mar;15(3):271-280. doi: 10.1513/AnnalsATS.201707-539OT. Ann Am Thorac Soc. 2018. PMID: 29342367
-
Ivacaftor as salvage therapy in a patient with cystic fibrosis genotype F508del/R117H/IVS8-5T.J Cyst Fibros. 2015 Jul;14(4):e4-5. doi: 10.1016/j.jcf.2015.01.010. Epub 2015 Feb 16. J Cyst Fibros. 2015. PMID: 25698453
-
F508del-cystic fibrosis transmembrane regulator correctors for treatment of cystic fibrosis: a patent review.Expert Opin Ther Pat. 2015;25(9):991-1002. doi: 10.1517/13543776.2015.1045878. Epub 2015 May 15. Expert Opin Ther Pat. 2015. PMID: 25971311 Review.
-
Cystic fibrosis transmembrane conductance regulator-modifying medications: the future of cystic fibrosis treatment.Ann Pharmacother. 2012 Jul-Aug;46(7-8):1065-75. doi: 10.1345/aph.1R076. Epub 2012 Jun 26. Ann Pharmacother. 2012. PMID: 22739718 Review.
Cited by
-
Lung Clearance Index Improves in People with Cystic Fibrosis not Achieving a Clinical Important Difference in Forced Expiratory Volume in One Second After Elexacaftor/Tezacaftor/Ivacaftor Therapy.Lung. 2024 Nov 30;203(1):9. doi: 10.1007/s00408-024-00768-1. Lung. 2024. PMID: 39614886
-
Real-world effectiveness of elexacaftor/tezacaftor/ivacaftor on the burden of illness in adolescents and adults with cystic fibrosis.Heliyon. 2024 Mar 26;10(7):e28508. doi: 10.1016/j.heliyon.2024.e28508. eCollection 2024 Apr 15. Heliyon. 2024. PMID: 38586424 Free PMC article.
-
Long-term impact of ivacaftor on mortality rate and health outcomes in people with cystic fibrosis.Thorax. 2024 Sep 18;79(10):925-933. doi: 10.1136/thorax-2023-220558. Thorax. 2024. PMID: 38937105 Free PMC article.
-
Long-Term Safety and Efficacy of Elexacaftor/Tezacaftor/Ivacaftor in Children Aged ⩾6 Years with Cystic Fibrosis and at Least One F508del Allele: A Phase 3, Open-Label Clinical Trial.Am J Respir Crit Care Med. 2023 Jul 1;208(1):68-78. doi: 10.1164/rccm.202301-0021OC. Am J Respir Crit Care Med. 2023. PMID: 37154609 Free PMC article.
References
-
- Jarzabek K, Zbucka M, Pepiński W, et al. . Cystic fibrosis as a cause of infertility. Reprod Biol 2004;4:119–29. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
