Phase-Synchronized Stimulus Presentation Augments Contingency Knowledge and Affective Evaluation in a Fear-Conditioning Task
- PMID: 34857589
- PMCID: PMC8751852
- DOI: 10.1523/ENEURO.0538-20.2021
Phase-Synchronized Stimulus Presentation Augments Contingency Knowledge and Affective Evaluation in a Fear-Conditioning Task
Abstract
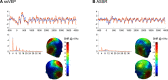
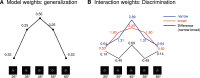
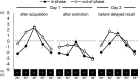
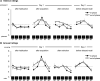
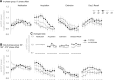
Memory often combines information from different sensory modalities. Animal studies show that synchronized neuronal activity in the theta band (4-8 Hz) binds multimodal associations. Studies with human participants have likewise established that theta-phase synchronization augments the formation of declarative video-tone pair memories. Another form of associative learning, classical fear conditioning, models nondeclarative, emotional memory with distinct neuronal mechanisms. Typical fear-conditioning tasks pair a conditioned stimulus (CS) in one modality with an aversive unconditioned stimulus (US) in another. The present study examines the effects of CS-US synchronization in the theta band on fear memory formation in humans. In a fear generalization procedure, we paired one of five visual gratings of varying orientation (CS) with an aversive auditory US. We modulated the luminance of the CS and the volume of the US at a rate of 4 Hz. To manipulate the synchrony between visual and auditory input during fear acquisition, one group (N = 20) received synchronous CS-US pairing, whereas the control group (N = 20) received the CS-US pairs out of phase. Phase synchronization improved CS-US contingency knowledge and facilitated CS discrimination in terms of rated valence and arousal, resulting in narrower generalization across the CS gratings compared with the out-of-phase group. In contrast, synchronization did not amplify conditioned responding in physiological arousal (skin conductance) and visuocortical engagement (steady-state visually evoked potentials) during acquisition, although both measures demonstrated tuning toward the CS+ Together, these data support a causal role of theta-phase synchronization in affective evaluation and contingency report during fear acquisition.
Keywords: associative memory; fear conditioning; multisensory; oscillations; phase synchronization; theta band.
Copyright © 2022 Plog et al.
Figures



Similar articles
-
Effects of phase synchronization and frequency specificity in the encoding of conditioned fear-a web-based fear conditioning study.PLoS One. 2023 Mar 3;18(3):e0281644. doi: 10.1371/journal.pone.0281644. eCollection 2023. PLoS One. 2023. PMID: 36867619 Free PMC article.
-
Learning pain-related fear: neural mechanisms mediating rapid differential conditioning, extinction and reinstatement processes in human visceral pain.Neurobiol Learn Mem. 2014 Dec;116:36-45. doi: 10.1016/j.nlm.2014.08.003. Epub 2014 Aug 13. Neurobiol Learn Mem. 2014. PMID: 25128878
-
Oscillatory EEG activity induced by conditioning stimuli during fear conditioning reflects Salience and Valence of these stimuli more than Expectancy.Neuroscience. 2017 Mar 27;346:81-93. doi: 10.1016/j.neuroscience.2016.12.047. Epub 2017 Jan 8. Neuroscience. 2017. PMID: 28077278 Free PMC article.
-
The need for standards in the design of differential fear conditioning and extinction experiments in youth: A systematic review and recommendations for research on anxiety.Behav Res Ther. 2019 Jan;112:42-62. doi: 10.1016/j.brat.2018.11.009. Epub 2018 Nov 20. Behav Res Ther. 2019. PMID: 30502721
-
Synaptic plasticity in the lateral amygdala: a cellular hypothesis of fear conditioning.Learn Mem. 2001 Sep-Oct;8(5):229-42. doi: 10.1101/lm.30901. Learn Mem. 2001. PMID: 11584069 Review.
Cited by
-
Effects of phase synchronization and frequency specificity in the encoding of conditioned fear-a web-based fear conditioning study.PLoS One. 2023 Mar 3;18(3):e0281644. doi: 10.1371/journal.pone.0281644. eCollection 2023. PLoS One. 2023. PMID: 36867619 Free PMC article.
-
Interaction between Theta Phase and Spike Timing-Dependent Plasticity Simulates Theta-Induced Memory Effects.eNeuro. 2023 Mar 13;10(3):ENEURO.0333-22.2023. doi: 10.1523/ENEURO.0333-22.2023. Print 2023 Mar. eNeuro. 2023. PMID: 36810147 Free PMC article.
-
The Relationship Between Self-Reported Misophonia Symptoms and Auditory Aversive Generalization Leaning: A Preliminary Report.Front Neurosci. 2022 Jun 23;16:899476. doi: 10.3389/fnins.2022.899476. eCollection 2022. Front Neurosci. 2022. PMID: 35812229 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
