Single-cell RNA sequencing reveals that BMPR2 mutation regulates right ventricular function via ID genes
- PMID: 34857612
- PMCID: PMC9260124
- DOI: 10.1183/13993003.00327-2021
Single-cell RNA sequencing reveals that BMPR2 mutation regulates right ventricular function via ID genes
Abstract
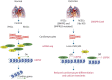
Background: Mutations in bone morphogenetic protein type II receptor (BMPR2) have been found in patients with congenital heart disease-associated pulmonary arterial hypertension (CHD-PAH). Our study aimed to clarify whether deficient BMPR2 signalling acts through downstream effectors, inhibitors of DNA-binding proteins (IDs) during heart development to contribute to the progress of PAH in CHD patients.
Methods: To confirm that IDs are downstream effectors of BMPR2 signalling in cardiac mesoderm progenitors (CMPs) and contribute to PAH, we generated cardiomyocyte-specific Id 1/3 knockout mice (Ids cDKO), and 12 out of 25 developed mild PAH with altered haemodynamic indices and pulmonary vascular remodelling. Moreover, we generated ID1 and ID3 double-knockout (IDs KO) human embryonic stem cells that recapitulated the BMPR2 signalling deficiency of CHD-PAH induced pluripotent stem cells (iPSCs).
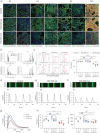
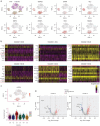
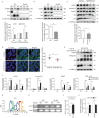
Results: Cardiomyocytes differentiated from iPSCs derived from CHD-PAH patients with BMP receptor mutations exhibited dysfunctional cardiac differentiation and reduced calcium (Ca2+) transients, as evidenced by confocal microscopy experiments. Smad1/5 phosphorylation and ID1 and ID3 expression were reduced in CHD-PAH iPSCs and in Bmpr2 +/- rat right ventricles. Moreover, ultrasound revealed that 33% of Ids cDKO mice had detectable defects in their ventricular septum and pulmonary regurgitation. Cardiomyocytes isolated from mouse right ventricles also showed reduced Ca2+ transients and shortened sarcomeres. Single-cell RNA sequencing analysis revealed impaired differentiation of CMPs and downregulated USP9X expression in IDs KO cells compared with wild-type cells.
Conclusion: We found that BMPR2 signals through IDs and USP9X to regulate cardiac differentiation, and the loss of ID1 and ID3 expression contributes to cardiomyocyte dysfunction in CHD-PAH patients with BMPR2 mutations.
Copyright ©The authors 2022.
Conflict of interest statement
Conflict of interest: N.W. Morrell reports grants and personal fees from Morphogen-IX, outside the submitted work; the other authors disclose no potential conflict of interest.
Figures





Similar articles
-
Bmpr2 Mutant Rats Develop Pulmonary and Cardiac Characteristics of Pulmonary Arterial Hypertension.Circulation. 2019 Feb 12;139(7):932-948. doi: 10.1161/CIRCULATIONAHA.118.033744. Circulation. 2019. PMID: 30586714
-
MiR-23a regulates the proliferation and migration of human pulmonary artery smooth muscle cells (HPASMCs) through targeting BMPR2/Smad1 signaling.Biomed Pharmacother. 2018 Jul;103:1279-1286. doi: 10.1016/j.biopha.2018.04.172. Epub 2018 May 7. Biomed Pharmacother. 2018. PMID: 29864909
-
Endothelin-Bone morphogenetic protein type 2 receptor interaction induces pulmonary artery smooth muscle cell hyperplasia in pulmonary arterial hypertension.J Heart Lung Transplant. 2015 Mar;34(3):468-78. doi: 10.1016/j.healun.2014.09.011. Epub 2014 Sep 28. J Heart Lung Transplant. 2015. PMID: 25447587
-
The Role of Bone Morphogenetic Protein Receptor Type 2 (BMPR2) and the Prospects of Utilizing Induced Pluripotent Stem Cells (iPSCs) in Pulmonary Arterial Hypertension Disease Modeling.Cells. 2022 Nov 29;11(23):3823. doi: 10.3390/cells11233823. Cells. 2022. PMID: 36497082 Free PMC article. Review.
-
Novel Advances in Modifying BMPR2 Signaling in PAH.Genes (Basel). 2020 Dec 23;12(1):8. doi: 10.3390/genes12010008. Genes (Basel). 2020. PMID: 33374819 Free PMC article. Review.
Cited by
-
CircGSAP alleviates pulmonary microvascular endothelial cells dysfunction in pulmonary hypertension via regulating miR-27a-3p/BMPR2 axis.Respir Res. 2022 Nov 19;23(1):322. doi: 10.1186/s12931-022-02248-7. Respir Res. 2022. PMID: 36403044 Free PMC article.
-
Deciphering the Cardiovascular Potential of Human CD34+ Stem Cells.Int J Mol Sci. 2023 May 31;24(11):9551. doi: 10.3390/ijms24119551. Int J Mol Sci. 2023. PMID: 37298503 Free PMC article.
-
Molecular remodeling in comorbidities associated with heart failure: a current update.Mol Biol Rep. 2024 Oct 26;51(1):1092. doi: 10.1007/s11033-024-10024-7. Mol Biol Rep. 2024. PMID: 39460797 Free PMC article. Review.
-
Stem cell therapy in pulmonary hypertension: current practice and future opportunities.Eur Respir Rev. 2023 Sep 27;32(169):230112. doi: 10.1183/16000617.0112-2023. Print 2023 Sep 30. Eur Respir Rev. 2023. PMID: 37758272 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
