Modulation of dopamine tone induces frequency shifts in cortico-basal ganglia beta oscillations
- PMID: 34857767
- PMCID: PMC8640051
- DOI: 10.1038/s41467-021-27375-5
Modulation of dopamine tone induces frequency shifts in cortico-basal ganglia beta oscillations
Abstract
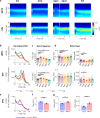
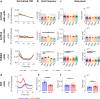
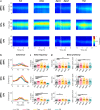
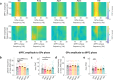
Βeta oscillatory activity (human: 13-35 Hz; primate: 8-24 Hz) is pervasive within the cortex and basal ganglia. Studies in Parkinson's disease patients and animal models suggest that beta-power increases with dopamine depletion. However, the exact relationship between oscillatory power, frequency and dopamine tone remains unclear. We recorded neural activity in the cortex and basal ganglia of healthy non-human primates while acutely and chronically up- and down-modulating dopamine levels. We assessed changes in beta oscillations in patients with Parkinson's following acute and chronic changes in dopamine tone. Here we show beta oscillation frequency is strongly coupled with dopamine tone in both monkeys and humans. Power, coherence between single-units and local field potentials (LFP), spike-LFP phase-locking, and phase-amplitude coupling are not systematically regulated by dopamine levels. These results demonstrate that beta frequency is a key property of pathological oscillations in cortical and basal ganglia networks.
© 2021. The Author(s).
Conflict of interest statement
R.E., Z.I., and H.B. declare the following competing interest: Activa PC + S pacemakers were donated by Medtronic (Medtronic Inc., Minneapolis, MN, USA). All other authors declare no competing interests.
Figures





Similar articles
-
Short- and long-term dopamine depletion causes enhanced beta oscillations in the cortico-basal ganglia loop of parkinsonian rats.Exp Neurol. 2016 Dec;286:124-136. doi: 10.1016/j.expneurol.2016.10.005. Epub 2016 Oct 12. Exp Neurol. 2016. PMID: 27743915
-
Changes in neuronal activity of cortico-basal ganglia-thalamic networks induced by acute dopaminergic manipulations in rats.Eur J Neurosci. 2018 Feb;47(3):236-250. doi: 10.1111/ejn.13805. Epub 2018 Jan 18. Eur J Neurosci. 2018. PMID: 29250896
-
Ventral Medial Thalamic Nucleus Promotes Synchronization of Increased High Beta Oscillatory Activity in the Basal Ganglia-Thalamocortical Network of the Hemiparkinsonian Rat.J Neurosci. 2016 Apr 13;36(15):4196-208. doi: 10.1523/JNEUROSCI.3582-15.2016. J Neurosci. 2016. PMID: 27076419 Free PMC article.
-
Basal ganglia oscillations and pathophysiology of movement disorders.Curr Opin Neurobiol. 2006 Dec;16(6):629-37. doi: 10.1016/j.conb.2006.10.002. Epub 2006 Nov 3. Curr Opin Neurobiol. 2006. PMID: 17084615 Review.
-
Changing views of the pathophysiology of Parkinsonism.Mov Disord. 2019 Aug;34(8):1130-1143. doi: 10.1002/mds.27741. Epub 2019 Jun 19. Mov Disord. 2019. PMID: 31216379 Review.
Cited by
-
Emergent Aspects of the Integration of Sensory and Motor Functions.Brain Sci. 2025 Feb 7;15(2):162. doi: 10.3390/brainsci15020162. Brain Sci. 2025. PMID: 40002495 Free PMC article. Review.
-
Shared pathway-specific network mechanisms of dopamine and deep brain stimulation for the treatment of Parkinson's disease.Nat Commun. 2025 Apr 15;16(1):3587. doi: 10.1038/s41467-025-58825-z. Nat Commun. 2025. PMID: 40234441 Free PMC article.
-
Beta-band power classification of go/no-go arm-reaching responses in the human hippocampus.J Neural Eng. 2024 Jul 15;21(4):046017. doi: 10.1088/1741-2552/ad5b19. J Neural Eng. 2024. PMID: 38914073 Free PMC article.
-
Changes in sensorimotor network dynamics in resting-state recordings in Parkinson's disease.Brain Commun. 2025 Jul 23;7(4):fcaf282. doi: 10.1093/braincomms/fcaf282. eCollection 2025. Brain Commun. 2025. PMID: 40761478 Free PMC article.
-
Single-neuron bursts encode pathological oscillations in subcortical nuclei of patients with Parkinson's disease and essential tremor.Proc Natl Acad Sci U S A. 2022 Aug 30;119(35):e2205881119. doi: 10.1073/pnas.2205881119. Epub 2022 Aug 26. Proc Natl Acad Sci U S A. 2022. PMID: 36018837 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

