Unique features of a recombinant haemagglutinin influenza vaccine that influence vaccine performance
- PMID: 34857771
- PMCID: PMC8640007
- DOI: 10.1038/s41541-021-00403-7
Unique features of a recombinant haemagglutinin influenza vaccine that influence vaccine performance
Abstract
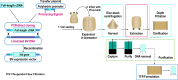
The influenza vaccine field has been constantly evolving to improve the speed, scalability, and flexibility of manufacturing, and to improve the breadth and longevity of the protective immune response across age groups, giving rise to an array of next generation vaccines in development. Among these, the recombinant influenza vaccine tetravalent (RIV4), using a baculovirus expression vector system to express recombinant haemagglutinin (rHA) in insect cells, is the only one to have reached the market and has been studied extensively. We describe how the unique structural features of rHA in RIV4 improve protective immune responses compared to conventional influenza vaccines made from propagated influenza virus. In addition to the sequence integrity, characteristic of recombinant proteins, unique post-translational processing of the rHA in insect cells instills favourable tertiary and quaternary structural features. The absence of protease-driven cleavage and addition of simple N-linked glycans help to preserve and expose certain conserved epitopes on HA molecules, which are likely responsible for the high levels of broadly cross-reactive and protective antibodies with rare specificities observed with RIV4. Furthermore, the presence of uniform compact HA oligomers and absence of egg proteins, viral RNA or process impurities, typically found in conventional vaccines, are expected to eliminate potential adverse reactions to these components in susceptible individuals with the use of RIV4. These distinct structural features and purity of the recombinant HA vaccine thus provide a number of benefits in vaccine performance which can be extended to other viral targets, such as for COVID-19.
© 2021. The Author(s).
Conflict of interest statement
All authors are employees of Sanofi Pasteur and may hold shares and/or stock options in the company.
Figures
References
-
- World Health Organization. Fact-sheet. Influenza (Seasonal). Available at https://www.who.int/en/news-room/fact-sheets/detail/influenza-(seasonal). Accessed 12 March 2021. (2018).
-
- US Centers for Disease Control and Prevention (CDC). Weekly U.S. Influenza Surveillance Report. https://www.cdc.gov/flu/weekly/index.htm. Accessed 16 March 2021.
Publication types
LinkOut - more resources
Full Text Sources

