Influenza virus vaccination in pediatric nephrotic syndrome significantly reduces rate of relapse and influenza virus infection as assessed in a nationwide survey
- PMID: 34857817
- PMCID: PMC8640023
- DOI: 10.1038/s41598-021-02644-x
Influenza virus vaccination in pediatric nephrotic syndrome significantly reduces rate of relapse and influenza virus infection as assessed in a nationwide survey
Abstract
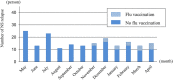
Although vaccination may precipitate relapses of nephrotic syndrome (NS) in children with idiopathic NS, no data are available regarding NS activity regarding influenza (flu) virus infections and NS relapses after receiving inactivated flu vaccines. We conducted a nationwide study of children aged 6 months to 15 years with idiopathic NS to assess the relationship between NS relapse, flu vaccination, and flu infections. We used a multivariate Poisson regression model (MPRM) to calculate the risk ratio (RR) for flu infection and for NS relapse in children with and without flu vaccination. Data of 306 children were assessed. The MPRM in all 306 children showed a significantly lower RR for flu infection (RR: 0.21, 95% confidence interval CI 0.11-0.38) and for NS relapse (RR: 0.22, 95% CI 0.14-0.35) in children receiving flu vaccination compared with unvaccinated children. In an additional MPRM only among 102 children receiving flu vaccination, they had a significantly lower risk for NS relapse during the post-vaccination period (RR: 0.31. 95% CI 017-0.56) compared with the pre-vaccination period. Although our study was observational, based on the favorable results of flu vaccinations regarding flu infections and NS relapse, the vaccine may be recommended for children with NS.
© 2021. The Author(s).
Conflict of interest statement
S.Is., T.A., K.K., C.T., M.S., F.K., R.H., Y.A., Y.G., K.N., H.N., T.M., N.Y. and M.H. have no financial relationships relevant to this article to disclose. Y.H. belonged to an endowed department sponsored by Asahi Kasei Pharma Corporation, Novartis Pharma K. K., Chugai Pharmaceutical Co., and Astellas Pharma (until 28 February 2018). K.Ii. received grants from Astellas Pharma, Daiichi Sankyo, and Zenyaku Kogyo. S.It. received grants from Asahi Kasei Pharma Corporation, Astellas Pharma, Chugai Pharmaceutical Co., Japan Blood Products Organization, Pfizer, and Teijin; and lecture and/or consulting fees from Asahi Kasei Pharma Corporation, Astellas Pharma, Chugai Pharmaceutical Co., Novartis Pharma K. K., and Zenyaku Kogyo. K.Is. received grants from Astellas Pharma, Daiichi Sankyo, and Zenyaku Kogyo.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

