Electrospun tube reduces adhesion in rabbit Achilles tendon 12 weeks post-surgery without PAR-2 overexpression
- PMID: 34857838
- PMCID: PMC8639666
- DOI: 10.1038/s41598-021-02780-4
Electrospun tube reduces adhesion in rabbit Achilles tendon 12 weeks post-surgery without PAR-2 overexpression
Abstract
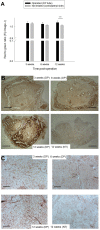
One great challenge in surgical tendon repair is the minimization of peritendinous adhesions. An electrospun tube can serve as a physical barrier around a conventionally sutured tendon. Six New Zealand White rabbits had one Achilles tendon fully transsected and sutured by a 4-strand suture. Another six rabbits had the same treatment, but with the additional electrospun DegraPol tube set around the sutured tendon. The adhesion formation to the surrounding tissue was investigated 12 weeks post-operation. Moreover, inflammation-related protease-activated receptor-2 (PAR-2) protein expression was assessed. Finally, rabbit Achilles tenocyte cultures were exposed to platelet-derived growth factor-BB (PDGF-BB), which mimicks the tendon healing environment, where PAR-2 gene expression was assessed as well as immunofluorescent staining intensity for F-actin and α-tubulin, respectively. At 12 weeks post-operation, the partially degraded DegraPol tube exhibited significantly lower adhesion formation (- 20%). PAR-2 protein expression was similar for time points 3 and 6 weeks, but increased at 12 weeks post-operation. In vitro cell culture experiments showed a significantly higher PAR-2 gene expression on day 3 after exposure to PDGF-BB, but not on day 7. The cytoskeleton of the tenocytes changed upon PDGF-BB stimulation, with signs of reorganization, and significantly decreased F-actin intensity. An electrospun DegraPol tube significantly reduces adhesion up to twelve weeks post-operation. At this time point, the tube is partially degraded, and a slight PAR-2 increase was detected in the DP treated tendons, which might however arise from particles of degrading DegraPol that were stained dark brown. PAR-2 gene expression in rabbit tenocytes reveals sensitivity at around day 10 after injury.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Serra CI, Navarro P, Guillem R, Soler C. Use of frozen tendon allograft in two clinical cases: Common calcaneal tendon and patellar ligament rupture. J. Am. Anim. Hosp. Assoc. 2020;56:315. - PubMed
-
- Elliot D, Giesen T. Primary flexor tendon surgery: The search for a perfect result. Hand Clin. 2013;29:191–206. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

