Heat stress reduces the contribution of diazotrophs to coral holobiont nitrogen cycling
- PMID: 34857934
- PMCID: PMC8941099
- DOI: 10.1038/s41396-021-01158-8
Heat stress reduces the contribution of diazotrophs to coral holobiont nitrogen cycling
Abstract
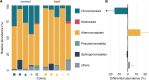
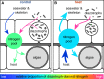
Efficient nutrient cycling in the coral-algal symbiosis requires constant but limited nitrogen availability. Coral-associated diazotrophs, i.e., prokaryotes capable of fixing dinitrogen, may thus support productivity in a stable coral-algal symbiosis but could contribute to its breakdown when overstimulated. However, the effects of environmental conditions on diazotroph communities and their interaction with other members of the coral holobiont remain poorly understood. Here we assessed the effects of heat stress on diazotroph diversity and their contribution to holobiont nutrient cycling in the reef-building coral Stylophora pistillata from the central Red Sea. In a stable symbiotic state, we found that nitrogen fixation by coral-associated diazotrophs constitutes a source of nitrogen to the algal symbionts. Heat stress caused an increase in nitrogen fixation concomitant with a change in diazotroph communities. Yet, this additional fixed nitrogen was not assimilated by the coral tissue or the algal symbionts. We conclude that although diazotrophs may support coral holobiont functioning under low nitrogen availability, altered nutrient cycling during heat stress abates the dependence of the coral host and its algal symbionts on diazotroph-derived nitrogen. Consequently, the role of nitrogen fixation in the coral holobiont is strongly dependent on its nutritional status and varies dynamically with environmental conditions.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Pogoreutz C, Voolstra CR, Rädecker N, Weis V, Cardenas A, Raina J-B. The coral holobiont highlights the dependence of cnidarian animal hosts on their associated microbes. In: Bosch TCG, Hadfield MG, editors. Cellular dialogues in the holobiont. CRC Press; 2020. p. 91–118.
-
- Stanley GD, van de Schootbrugge B. The evolution of the coral–algal symbiosis. In: van Oppen MJH, Lough JM, editors. Coral bleaching: patterns, processes, causes and consequences. Berlin, Heidelberg: Springer; 2009. p. 7–19.
-
- LaJeunesse TC, Parkinson JE, Gabrielson PW, Jeong HJ, Reimer JD, Voolstra CR, et al. Systematic revision of Symbiodiniaceae highlights the antiquity and diversity of coral endosymbionts. Curr Biol. 2018;28:2570–80.e6. - PubMed
-
- Muscatine L. The role of symbiotic algae in carbon and energy flux in reef corals. Coral Reefs. 1990;25:75–87.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

