Comparison of Risk of Pneumonia Caused by Fluticasone Propionate versus Budesonide in Chronic Obstructive Pulmonary Disease: A Nationwide Retrospective Cohort Study
- PMID: 34858023
- PMCID: PMC8629914
- DOI: 10.2147/COPD.S332151
Comparison of Risk of Pneumonia Caused by Fluticasone Propionate versus Budesonide in Chronic Obstructive Pulmonary Disease: A Nationwide Retrospective Cohort Study
Abstract
Introduction: Inhaled corticosteroids (ICSs) play an important role in lowering the risk of acute exacerbation of chronic obstructive pulmonary disease (COPD). However, ICSs are known to increase the risk of pneumonia. Moreover, previous studies have shown that the incidence rate of pneumonia varies depending on the type of ICS. In this study, the risk of pneumonia according to the type of ICS was investigated in a population-based cohort.
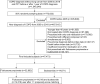
Methods: A retrospective cohort study was conducted using claims data of the entire population from the Korean National Health Insurance Service. Patients who were newly diagnosed with COPD and prescribed fluticasone propionate or budesonide were enrolled as study subjects. Cumulative doses of ICSs were classified into categorical variables to analyze the risk of pneumonia within identical ICS doses.
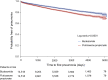
Results: A total of 47,473 subjects were identified and allocated as 14,518 fluticasone propionate and 14,518 budesonide users through 1:1 propensity score matching. Fluticasone propionate users were more likely to develop pneumonia than budesonide users (14.22% vs 10.66%, p<0.0001). The incidence rate per 100,000 person-years was 2,914.77 for fluticasone propionate users and 2,102.90 for budesonide users. The hazard ratio (HR) of pneumonia in fluticasone propionate compared to budesonide was 1.34 (95% CI 1.26-1.43, p<0.0001). The risk of pneumonia for fluticasone propionate compared to budesonide increased with higher ICS cumulative doses: 1.06 (0.93-1.21), 1.41 (1.19-1.66), 1.41 (1.23-1.63), and 1.49 (1.33-1.66) from the lowest to highest quartiles, respectively.
Conclusion: ICS types and doses need to be carefully considered during treatment with ICSs in patients with COPD.
Keywords: budesonide; chronic obstructive pulmonary disease; fluticasone propionate; inhaled corticosteroid; pneumonia.
© 2021 Choi et al.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Risk of Pneumonia Associated with Inhaled Corticosteroid in Patients with Chronic Obstructive Pulmonary Disease: A Korean Population-Based Study.Int J Chron Obstruct Pulmon Dis. 2020 Dec 29;15:3397-3406. doi: 10.2147/COPD.S286149. eCollection 2020. Int J Chron Obstruct Pulmon Dis. 2020. PMID: 33402820 Free PMC article.
-
Intraclass Difference in Pneumonia Risk with Fluticasone and Budesonide in COPD: A Systematic Review of Evidence from Direct-Comparison Studies.Int J Chron Obstruct Pulmon Dis. 2020 Nov 11;15:2889-2900. doi: 10.2147/COPD.S269637. eCollection 2020. Int J Chron Obstruct Pulmon Dis. 2020. PMID: 33204085 Free PMC article.
-
Risk of Tuberculosis Caused by Fluticasone Propionate versus Budesonide in Chronic Obstructive Pulmonary Disease: A Nationwide Population-Based Study.J Pers Med. 2022 Jul 21;12(7):1189. doi: 10.3390/jpm12071189. J Pers Med. 2022. PMID: 35887686 Free PMC article.
-
The association between inhaled corticosteroid and pneumonia in COPD patients: the improvement of patients' life quality with COPD in Taiwan (IMPACT) study.Int J Chron Obstruct Pulmon Dis. 2016 Nov 8;11:2775-2783. doi: 10.2147/COPD.S116750. eCollection 2016. Int J Chron Obstruct Pulmon Dis. 2016. PMID: 27877031 Free PMC article.
-
Scientific rationale for the possible inhaled corticosteroid intraclass difference in the risk of pneumonia in COPD.Int J Chron Obstruct Pulmon Dis. 2017 Oct 19;12:3055-3064. doi: 10.2147/COPD.S143656. eCollection 2017. Int J Chron Obstruct Pulmon Dis. 2017. PMID: 29089754 Free PMC article. Review.
Cited by
-
Eosinophil count and treatment response in COPD patients.Lung India. 2025 Jul 1;42(4):337-342. doi: 10.4103/lungindia.lungindia_630_24. Epub 2025 Jun 27. Lung India. 2025. PMID: 40569402 Free PMC article.
-
Exploring the appropriateness of prescribing practice of inhaled pharmacotherapy among Aboriginal Australians in the Top End Northern Territory of Australia: a retrospective cohort study.BMJ Open Respir Res. 2023 Mar;10(1):e001508. doi: 10.1136/bmjresp-2022-001508. BMJ Open Respir Res. 2023. PMID: 36878611 Free PMC article.
-
Budesonide-Formoterol Metered-Dose Inhaler vs Fluticasone-Salmeterol Dry-Powder Inhaler.JAMA Intern Med. 2025 Aug 1;185(8):1005-1013. doi: 10.1001/jamainternmed.2025.2299. JAMA Intern Med. 2025. PMID: 40622686 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

