Serum Untargeted UHPLC-HRMS-Based Lipidomics to Discover the Potential Biomarker of Colorectal Advanced Adenoma
- PMID: 34858060
- PMCID: PMC8632617
- DOI: 10.2147/CMAR.S336322
Serum Untargeted UHPLC-HRMS-Based Lipidomics to Discover the Potential Biomarker of Colorectal Advanced Adenoma
Abstract
Background: As a key precancerous lesion, colorectal advanced adenoma (CAA) is closely related to the occurrence and development of colorectal cancer (CRC). Effective identification of CAA-related biomarkers can prevent CRC morbidity and mortality. Lipids, as an important endogenous substance, have been proved to be involved in the occurrence and development of CRC. Lipidomics is an advanced technique that studies lipid metabolism and biomarkers of diseases. However, there are no lipidomics studies based on large serum samples to explore diagnostic biomarkers for CAA.
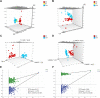
Methods: An integrated serum lipid profile from 50 normal (NR) and 46 CAA subjects was performed using ultra-high performance liquid chromatography tandem high-resolution mass spectrometry (UHPLC-HRMS). Lipidomic data were acquired for negative and positive ionization modes, respectively. Differential lipids were selected by univariate and multivariate statistics analyses. A receiver operator characteristic curve (ROC) analysis was conducted to evaluate the diagnostic performance of differential lipids.
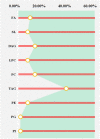
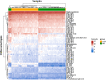
Results: A total of 53 differential lipids were obtained by combining univariate and multivariate statistical analyses (P < 0.05 and VIP > 1). In addition, 12 differential lipids showed good diagnostic performance (AUC > 0.90) for the discrimination of NR and CAA by receiver operating characteristic curve (ROC) analysis. Of them, the performance of PC 44:5 and PC 35:6e presented the outstanding performance (AUC = 1.00, (95% CI, 1.00-1.00)). Moreover, triglyceride (TAG) had the highest proportion (37.74%) as the major dysregulated lipids in the CAA.
Conclusion: This is the first study that profiled serum lipidomics and explored lipid biomarkers with good diagnostic ability of CAA to contribute to the early prevention of CRC. Twelve differential lipids that effectively discriminate between NR and CAA serve as the potential diagnostic markers of CAA. An obvious perturbation of TAG metabolism could be involved in the CAA formation.
Keywords: UHPLC-HRMS; biomarker; colorectal advanced adenoma; colorectal cancer; serum lipidomics.
© 2021 Zhu et al.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





Similar articles
-
Serum untargeted lipidomics by UHPLC-ESI-HRMS aids the biomarker discovery of colorectal adenoma.BMC Cancer. 2022 Mar 24;22(1):314. doi: 10.1186/s12885-022-09427-1. BMC Cancer. 2022. PMID: 35331175 Free PMC article.
-
UHPLC-HRMS-based serum untargeted lipidomics: Phosphatidylcholines and sphingomyelins are the main disturbed lipid markers to distinguish colorectal advanced adenoma from cancer.J Pharm Biomed Anal. 2023 Sep 20;234:115582. doi: 10.1016/j.jpba.2023.115582. Epub 2023 Jul 16. J Pharm Biomed Anal. 2023. PMID: 37473505
-
UHPLC-HRMS-based Multiomics to Explore the Potential Mechanisms and Biomarkers for Colorectal Cancer.BMC Cancer. 2024 May 27;24(1):644. doi: 10.1186/s12885-024-12321-7. BMC Cancer. 2024. PMID: 38802800 Free PMC article.
-
Advances in lipidomics.Clin Chim Acta. 2020 Nov;510:123-141. doi: 10.1016/j.cca.2020.06.049. Epub 2020 Jul 3. Clin Chim Acta. 2020. PMID: 32622966 Review.
-
Ultra-performance liquid chromatography-mass spectrometry as a sensitive and powerful technology in lipidomic applications.Chem Biol Interact. 2014 Sep 5;220:181-92. doi: 10.1016/j.cbi.2014.06.029. Epub 2014 Jul 9. Chem Biol Interact. 2014. PMID: 25014415 Review.
Cited by
-
Serum untargeted lipidomics by UHPLC-ESI-HRMS aids the biomarker discovery of colorectal adenoma.BMC Cancer. 2022 Mar 24;22(1):314. doi: 10.1186/s12885-022-09427-1. BMC Cancer. 2022. PMID: 35331175 Free PMC article.
-
The Utility of Lipidomic Analysis in Colorectal Cancer Diagnosis and Prognosis-A Systematic Review of Recent Literature.Int J Mol Sci. 2024 Jul 14;25(14):7722. doi: 10.3390/ijms25147722. Int J Mol Sci. 2024. PMID: 39062964 Free PMC article.
-
Lipid Biomarkers in Liquid Biopsies: Novel Opportunities for Cancer Diagnosis.Pharmaceutics. 2023 Jan 28;15(2):437. doi: 10.3390/pharmaceutics15020437. Pharmaceutics. 2023. PMID: 36839759 Free PMC article. Review.
-
[Integrated analysis of serum untargeted metabolomics and targeted bile acid metabolomics for identification of diagnostic biomarkers for colorectal cancer].Nan Fang Yi Ke Da Xue Xue Bao. 2023 Mar 20;43(3):443-453. doi: 10.12122/j.issn.1673-4254.2023.03.15. Nan Fang Yi Ke Da Xue Xue Bao. 2023. PMID: 37087590 Free PMC article. Chinese.
-
UHPLC-HRMS-based serum lipisdomics reveals novel biomarkers to assist in the discrimination between colorectal adenoma and cancer.Front Oncol. 2022 Jul 28;12:934145. doi: 10.3389/fonc.2022.934145. eCollection 2022. Front Oncol. 2022. PMID: 35965551 Free PMC article.
References
-
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
-
- Aepli P, Criblez D, Baumeler S, et al. Endoscopic full thickness resection (EFTR) of colorectal neoplasms with the Full Thickness Resection Device (FTRD): clinical experience from two tertiary referral centers in Switzerland. United Eur Gastroenterol J. 2018;6:463–470. doi:10.1177/2050640617728001 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

