Electroencephalogram-Based Complexity Measures as Predictors of Post-operative Neurocognitive Dysfunction
- PMID: 34858144
- PMCID: PMC8631543
- DOI: 10.3389/fnsys.2021.718769
Electroencephalogram-Based Complexity Measures as Predictors of Post-operative Neurocognitive Dysfunction
Abstract
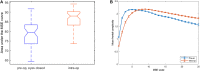
Physiologic signals such as the electroencephalogram (EEG) demonstrate irregular behaviors due to the interaction of multiple control processes operating over different time scales. The complexity of this behavior can be quantified using multi-scale entropy (MSE). High physiologic complexity denotes health, and a loss of complexity can predict adverse outcomes. Since postoperative delirium is particularly hard to predict, we investigated whether the complexity of preoperative and intraoperative frontal EEG signals could predict postoperative delirium and its endophenotype, inattention. To calculate MSE, the sample entropy of EEG recordings was computed at different time scales, then plotted against scale; complexity is the total area under the curve. MSE of frontal EEG recordings was computed in 50 patients ≥ age 60 before and during surgery. Average MSE was higher intra-operatively than pre-operatively (p = 0.0003). However, intraoperative EEG MSE was lower than preoperative MSE at smaller scales, but higher at larger scales (interaction p < 0.001), creating a crossover point where, by definition, preoperative, and intraoperative MSE curves met. Overall, EEG complexity was not associated with delirium or attention. In 42/50 patients with single crossover points, the scale at which the intraoperative and preoperative entropy curves crossed showed an inverse relationship with delirium-severity score change (Spearman ρ = -0.31, p = 0.054). Thus, average EEG complexity increases intra-operatively in older adults, but is scale dependent. The scale at which preoperative and intraoperative complexity is equal (i.e., the crossover point) may predict delirium. Future studies should assess whether the crossover point represents changes in neural control mechanisms that predispose patients to postoperative delirium.
Keywords: anesthesia; attention; cognition; complexity; delirium; electroencephalogram (EEG); perioperative medicine; resilience.
Copyright © 2021 Acker, Ha, Zhou, Manor, Giattino, Roberts, Berger, Wright, Colon-Emeric, Devinney, Au, Woldorff, Lipsitz and Whitson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Babiloni C., Lizio R., Marzano N., Capotosto P., Soricelli A., Triggiani A. I., et al. (2016). Brain neural synchronization and functional coupling in Alzheimer’s disease as revealed by resting state EEG rhythms. Int. J. Psychophysiol. 103 88–102. - PubMed
-
- Berger M., Browndyke J., Mathew J. P. (2015a). Intraoperative glycemic control to prevent delirium after cardiac surgery: steering a course between scylla and charybdis. Anesthesiology 122 1186–1188. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

