Antioxidant Potential and Inhibition of Mitochondrial Permeability Transition Pore by Myricetin Reduces Aluminium Phosphide-Induced Cytotoxicity and Mitochondrial Impairments
- PMID: 34858168
- PMCID: PMC8630626
- DOI: 10.3389/fphar.2021.719081
Antioxidant Potential and Inhibition of Mitochondrial Permeability Transition Pore by Myricetin Reduces Aluminium Phosphide-Induced Cytotoxicity and Mitochondrial Impairments
Abstract
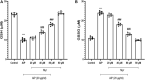
Oxidative stress and mitochondrial dysfunction are involved in the mechanisms of cardiac toxicity induced by aluminum phosphide (AlP). AlP-induced cardiotoxicity leads to cardiomyocyte death, cardiomyopathy, cardiac dysfunction, and eventually severe heart failure and death. Importantly, protecting cardiomyocytes from death resulting from AlP is vital for improving survival. It has been reported that flavonoids such as myricetin (Myr) act as modifiers of mitochondrial function and prevent mitochondrial damage resulting from many insults and subsequent cell dysfunction. In this study, the ameliorative effect of Myr, as an important antioxidant and mitochondrial protective agent, was investigated in cardiomyocytes and mitochondria isolated from rat heart against AlP-induced toxicity, oxidative stress, and mitochondrial dysfunction. Treatment of AlP (20 μg/ml) significantly increased cytotoxicity; reduced glutathione (GSH) depletion, cellular reactive oxygen species (ROS) formation, malondialdehyde (MDA) level, ATP depletion, caspase-3 activation, mitochondrial membrane potential (ΔΨm) collapse, and lysosomal dysfunction; and decreased the activities of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px) in intact cardiomyocytes. Also, treatment of AlP (20 μg/ml) significantly increased mitochondrial dysfunction and swelling in isolated mitochondria. Myr (80 µM) appeared to ameliorate AlP-induced cytotoxicity in isolated cardiomyocytes; significantly lessened the AlP-stimulated intracellular ROS and MDA production and depletion of GSH; and increased the activities of SOD, CAT, and GSH-Px. Furthermore, Myr (40 and 80 µM) lowered AlP-induced lysosomal/mitochondrial dysfunction, ATP depletion, and caspase-3 activation. In the light of these findings, we concluded that Myr through antioxidant potential and inhibition of mitochondrial permeability transition (MPT) pore exerted an ameliorative role in AlP-induced toxicity in isolated cardiomyocytes and mitochondria, and it would be valuable to examine its in vivo effects.
Keywords: antioxidant; cardiomyopathy; flavonoids; mitochondrial dysfunction; poisoning.
Copyright © 2021 Salimi, Jamali and Shabani.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Anand R., Sharma D. R., Verma D., Bhalla A., Gill K. D., Singh S. (2013). Mitochondrial Electron Transport Chain Complexes, Catalase and Markers of Oxidative Stress in Platelets of Patients with Severe Aluminum Phosphide Poisoning. Hum. Exp. Toxicol. 32, 807–816. 10.1177/0960327112468909 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

