Urinary mRNA Signatures as Predictors of Renal Function Decline in Patients With Biopsy-Proven Diabetic Kidney Disease
- PMID: 34858345
- PMCID: PMC8630698
- DOI: 10.3389/fendo.2021.774436
Urinary mRNA Signatures as Predictors of Renal Function Decline in Patients With Biopsy-Proven Diabetic Kidney Disease
Abstract
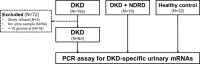
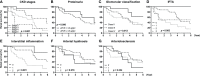
The clinical manifestations of diabetic kidney disease (DKD) are more heterogeneous than those previously reported, and these observations mandate the need for the recruitment of patients with biopsy-proven DKD in biomarker research. In this study, using the public gene expression omnibus (GEO) repository, we aimed to identify urinary mRNA biomarkers that can predict histological severity and disease progression in patients with DKD in whom the diagnosis and histologic grade has been confirmed by kidney biopsy. We identified 30 DKD-specific mRNA candidates based on the analysis of the GEO datasets. Among these, there were significant alterations in the urinary levels of 17 mRNAs in patients with DKD, compared with healthy controls. Four urinary mRNAs-LYZ, C3, FKBP5, and G6PC-reflected tubulointerstitial inflammation and fibrosis in kidney biopsy and could predict rapid progression to end-stage kidney disease independently of the baseline eGFR (tertile 1 vs. tertile 3; adjusted hazard ratio of 9.68 and 95% confidence interval of 2.85-32.87, p < 0.001). In conclusion, we demonstrated that urinary mRNA signatures have a potential to indicate the pathologic status and predict adverse renal outcomes in patients with DKD.
Keywords: biomarker; diabetic kidney disease; mRNA; renal pathology; urine.
Copyright © 2021 Lee, Seo, Kim, Tae, Seok, Kim, Lee, Kim, Hwang, Jeong and Moon.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Association of urinary acidification function with the progression of diabetic kidney disease in patients with type 2 diabetes.J Diabetes Complications. 2019 Nov;33(11):107419. doi: 10.1016/j.jdiacomp.2019.107419. Epub 2019 Aug 19. J Diabetes Complications. 2019. PMID: 31473080
-
Uromodulin mRNA from Urinary Extracellular Vesicles Correlate to Kidney Function Decline in Type 2 Diabetes Mellitus.Am J Nephrol. 2018;47(5):283-291. doi: 10.1159/000489129. Epub 2018 May 18. Am J Nephrol. 2018. PMID: 29779026
-
Serum hemoglobin concentration and risk of renal function decline in early stages of diabetic kidney disease: a nationwide, biopsy-based cohort study.Nephrol Dial Transplant. 2022 Feb 25;37(3):489-497. doi: 10.1093/ndt/gfab185. Nephrol Dial Transplant. 2022. PMID: 34028524
-
Novel biomarkers for prognosticating diabetic kidney disease progression.Int Urol Nephrol. 2023 Apr;55(4):913-928. doi: 10.1007/s11255-022-03354-7. Epub 2022 Oct 22. Int Urol Nephrol. 2023. PMID: 36271990 Free PMC article. Review.
-
[Research status and prospect of novel biomarkers for diabetic kidney disease].Zhonghua Yi Xue Za Zhi. 2021 Mar 16;101(10):691-694. doi: 10.3760/cma.j.cn112137-20201110-03055. Zhonghua Yi Xue Za Zhi. 2021. PMID: 33721945 Review. Chinese.
Cited by
-
Identification and validation of voltage-dependent anion channel 1-related genes and immune cell infiltration in diabetic nephropathy.J Diabetes Investig. 2024 Jan;15(1):87-105. doi: 10.1111/jdi.14087. Epub 2023 Sep 22. J Diabetes Investig. 2024. PMID: 37737517 Free PMC article.
-
Cellular and Functional Biomarkers of Renal Injury and Disease.Curr Opin Toxicol. 2022 Sep;31:100348. doi: 10.1016/j.cotox.2022.100348. Epub 2022 May 6. Curr Opin Toxicol. 2022. PMID: 36540379 Free PMC article.
-
Plantaginis Semen Ameliorates Hyperuricemia Induced by Potassium Oxonate.Int J Mol Sci. 2024 Aug 5;25(15):8548. doi: 10.3390/ijms25158548. Int J Mol Sci. 2024. PMID: 39126116 Free PMC article.
-
Intersecting transcriptomic landscapes of hypertension and kidney function in African American women.Am J Physiol Renal Physiol. 2025 Jul 1;329(1):F59-F70. doi: 10.1152/ajprenal.00067.2025. Epub 2025 May 30. Am J Physiol Renal Physiol. 2025. PMID: 40445960 Free PMC article.
-
Identification of endoplasmic reticulum stress-related biomarkers of diabetes nephropathy based on bioinformatics and machine learning.Front Endocrinol (Lausanne). 2023 Sep 1;14:1206154. doi: 10.3389/fendo.2023.1206154. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37745718 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

