β2 Integrin CD11d/CD18: From Expression to an Emerging Role in Staged Leukocyte Migration
- PMID: 34858434
- PMCID: PMC8630586
- DOI: 10.3389/fimmu.2021.775447
β2 Integrin CD11d/CD18: From Expression to an Emerging Role in Staged Leukocyte Migration
Abstract
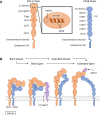
CD11d/CD18 is the most recently discovered and least understood β2 integrin. Known CD11d adhesive mechanisms contribute to both extravasation and mesenchymal migration - two key aspects for localizing peripheral leukocytes to sites of inflammation. Differential expression of CD11d induces differences in monocyte/macrophage mesenchymal migration including impacts on macrophage sub-set migration. The participation of CD11d/CD18 in leukocyte localization during atherosclerosis and following neurotrauma has sparked interest in the development of CD11d-targeted therapeutic agents. Whereas the adhesive properties of CD11d have undergone investigation, the signalling pathways induced by ligand binding remain largely undefined. Underlining each adhesive and signalling function, CD11d is under unique transcriptional control and expressed on a sub-set of predominately tissue-differentiated innate leukocytes. The following review is the first to capture the nearly three decades of CD11d research and discusses the emerging role of CD11d in leukocyte migration and retention during the progression of a staged immune response.
Keywords: CD11d; CD18; beta 2 integrin; extravasation; inflammation; leukocyte; migration.
Copyright © 2021 Blythe, Weaver, Brown and Dekaban.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Danilenko DM, Rossitto PV, Vieren MVd, Trong HL, McDonough SP, Affolter VK, et al. . A Novel Canine Leukointegrin, Alpha D Beta 2, Is Expressed by Specific Macrophage Subpopulations in Tissue and a Minor CD8+ Lymphocyte Subpopulation in Peripheral Blood. J Immunol (1995) 155:35–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

