Physiological and Transcriptomic Analyses Revealed the Implications of Abscisic Acid in Mediating the Rate-Limiting Step for Photosynthetic Carbon Dioxide Utilisation in Response to Vapour Pressure Deficit in Solanum Lycopersicum (Tomato)
- PMID: 34858453
- PMCID: PMC8631768
- DOI: 10.3389/fpls.2021.745110
Physiological and Transcriptomic Analyses Revealed the Implications of Abscisic Acid in Mediating the Rate-Limiting Step for Photosynthetic Carbon Dioxide Utilisation in Response to Vapour Pressure Deficit in Solanum Lycopersicum (Tomato)
Abstract
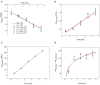
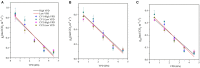
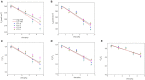
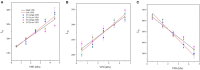
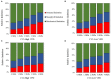
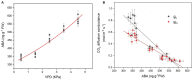
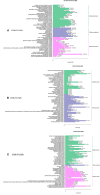
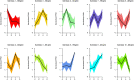
The atmospheric vapour pressure deficit (VPD) has been demonstrated to be a significant environmental factor inducing plant water stress and affecting plant photosynthetic productivity. Despite this, the rate-limiting step for photosynthesis under varying VPD is still unclear. In the present study, tomato plants were cultivated under two contrasting VPD levels: high VPD (3-5 kPa) and low VPD (0.5-1.5 kPa). The effect of long-term acclimation on the short-term rapid VPD response was examined across VPD ranging from 0.5 to 4.5 kPa. Quantitative photosynthetic limitation analysis across the VPD range was performed by combining gas exchange and chlorophyll fluorescence. The potential role of abscisic acid (ABA) in mediating photosynthetic carbon dioxide (CO2) uptake across a series of VPD was evaluated by physiological and transcriptomic analyses. The rate-limiting step for photosynthetic CO2 utilisation varied with VPD elevation in tomato plants. Under low VPD conditions, stomatal and mesophyll conductance was sufficiently high for CO2 transport. With VPD elevation, plant water stress was gradually pronounced and triggered rapid ABA biosynthesis. The contribution of stomatal and mesophyll limitation to photosynthesis gradually increased with an increase in the VPD. Consequently, the low CO2 availability inside chloroplasts substantially constrained photosynthesis under high VPD conditions. The foliar ABA content was negatively correlated with stomatal and mesophyll conductance for CO2 diffusion. Transcriptomic and physiological analyses revealed that ABA was potentially involved in mediating water transport and photosynthetic CO2 uptake in response to VPD variation. The present study provided new insights into the underlying mechanism of photosynthetic depression under high VPD stress.
Keywords: abscisic acid; evaporative demand; mesophyll conductance; plant water status; stomatal conductance.
Copyright © 2021 Zhang, Du, Sun, Lou, Li, Li and Wei.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources

