Efficacy and Safety of Lianhuaqingwen Capsules for the Prevention of Coronavirus Disease 2019: A Prospective Open-Label Controlled Trial
- PMID: 34858512
- PMCID: PMC8632393
- DOI: 10.1155/2021/7962630
Efficacy and Safety of Lianhuaqingwen Capsules for the Prevention of Coronavirus Disease 2019: A Prospective Open-Label Controlled Trial
Abstract
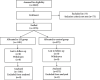
Coronavirus disease 2019 (COVID-19) has become a global pandemic. Community and close contact exposures continue to drive the COVID-19 pandemic. There is no confirmed effective treatment for suspected cases and close contacts. Lianhuaqingwen (LH) capsules, a repurposed Chinese herbal product that is currently on the market, have proven effective for influenza and COVID-19. To determine the safety and efficacy of LH capsules for the prevention of COVID-19, we conducted a prospective open-label controlled trial of LH capsules on subjects who had close contact with people infected with COVID-19. Subjects received LH capsules (4 capsules, three times daily) or the usual medical observation for 14 days. The primary endpoint was the rate of positive nucleic acid tests of nasal and pharyngeal swabs during the quarantine medical observation period. We included 1976 patients, including 1101 in the treatment group and 875 in the control group. The rate of positive nucleic acid tests in the treatment group was significantly lower than that in the control group (0.27% vs. 1.14%, respectively; mean difference: -0.87%; 95% CI: -1.83 to -0.13; p=0.0174) during the quarantine medical observation period (14 days). Among subjects with different close contact states, there was no significant difference in the rate of positive nucleic acid test results among close contacts in the treatment group and the control group (6.45% vs. 11.43%, respectively; p=0.6762). Among secondary close contacts, the rate of positive nucleic acid tests in the treatment group was significantly lower than that in the control group (0.09% vs. 0.71%, respectively; p=0.0485). No serious adverse events were reported. Taken together, and in light of the safety and effectiveness profiles, these results show that LH capsules can be considered to prevent the progression of COVID-19 after close contact with an infected person. This trial is registered with ChiCTR2100043012.
Copyright © 2021 Xiaowei Gong et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest. Yiling Pharmaceutical Co., Ltd. provided the medications for the study, while they did not participate in research design, data collection, data analysis, data interpretation, nor article writing.
Figures
Similar articles
-
Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: A multicenter, prospective, randomized controlled trial.Phytomedicine. 2021 May;85:153242. doi: 10.1016/j.phymed.2020.153242. Epub 2020 May 16. Phytomedicine. 2021. PMID: 33867046 Free PMC article. Clinical Trial.
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Effect of Lianhua Qingwen capsules on the positive rate of COVID-19 close contacts: A retrospective analysis of a large-scale population-based cohort study.Phytomedicine. 2023 Apr;112:154690. doi: 10.1016/j.phymed.2023.154690. Epub 2023 Feb 3. Phytomedicine. 2023. PMID: 36780823 Free PMC article.
-
Efficacy and safety of herbal medicine (Lianhuaqingwen) for treating COVID-19: A systematic review and meta-analysis.Integr Med Res. 2021 Mar;10(1):100644. doi: 10.1016/j.imr.2020.100644. Epub 2020 Aug 21. Integr Med Res. 2021. PMID: 32864332 Free PMC article. Review.
-
Travel-related control measures to contain the COVID-19 pandemic: a rapid review.Cochrane Database Syst Rev. 2020 Oct 5;10:CD013717. doi: 10.1002/14651858.CD013717. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Mar 25;3:CD013717. doi: 10.1002/14651858.CD013717.pub2. PMID: 33502002 Updated.
Cited by
-
Comprehensive characterization of the chemical constituents of Lianhua Qingwen capsule by ultra high performance liquid chromatography coupled with Fourier transform ion cyclotron resonance mass spectrometry.Heliyon. 2024 Mar 3;10(6):e27352. doi: 10.1016/j.heliyon.2024.e27352. eCollection 2024 Mar 30. Heliyon. 2024. PMID: 38496865 Free PMC article.
-
Efficacy and safety of Lianhua Qingwen as an adjuvant treatment for influenza in Chinese patients: A meta-analysis.Medicine (Baltimore). 2024 Jan 19;103(3):e36986. doi: 10.1097/MD.0000000000036986. Medicine (Baltimore). 2024. PMID: 38241551 Free PMC article.
-
Efficacy and safety of Chinese herbal medicine to prevent and treat COVID-19 household close contacts in Hong Kong: an open-label, randomized controlled trial.Front Immunol. 2024 May 10;15:1359331. doi: 10.3389/fimmu.2024.1359331. eCollection 2024. Front Immunol. 2024. PMID: 38799438 Free PMC article. Clinical Trial.
-
Efficacy and safety of Lianhua Qingwen capsules combined with standard of care in the treatment of adult patients with mild to moderate COVID-19 (FLOSAN): protocol for a randomized, double-blind, international multicenter clinical trial.J Thorac Dis. 2023 May 30;15(5):2859-2872. doi: 10.21037/jtd-23-281. Epub 2023 Apr 23. J Thorac Dis. 2023. PMID: 37324081 Free PMC article.
-
Effects of Lianhuaqingwen Capsules in adults with mild-to-moderate coronavirus disease 2019: an international, multicenter, double-blind, randomized controlled trial.Virol J. 2023 Nov 28;20(1):277. doi: 10.1186/s12985-023-02144-6. Virol J. 2023. PMID: 38017515 Free PMC article. Clinical Trial.
References
-
- National Health Commission State Administration of Traditional Chinese Medicine. National Health Commission of the People’s Republic of China. Chinese management guideline for COVID-19(version 8.0) Infectious Disease Information . 2020;33(4):289–296.
LinkOut - more resources
Full Text Sources