A brief review on DNA vaccines in the era of COVID-19
- PMID: 34858516
- PMCID: PMC8629371
- DOI: 10.2217/fvl-2021-0170
A brief review on DNA vaccines in the era of COVID-19
Abstract
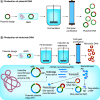
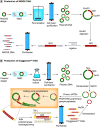
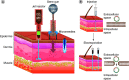
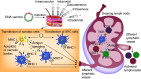
This article provides a brief overview of DNA vaccines. First, the basic DNA vaccine design strategies are described, then specific issues related to the industrial production of DNA vaccines are discussed, including the production and purification of DNA products such as plasmid DNA, minicircle DNA, minimalistic, immunologically defined gene expression (MIDGE) and Doggybone™. The use of adjuvants to enhance the immunogenicity of DNA vaccines is then discussed. In addition, different delivery routes and several physical and chemical methods to increase the efficacy of DNA delivery into cells are explained. Recent preclinical and clinical trials of DNA vaccines for COVID-19 are then summarized. Lastly, the advantages and obstacles of DNA vaccines are discussed.
Keywords: COVID-19; DNA; DNA vaccines; SARS-CoV-2; nucleic acid vaccines; vaccines.
© 2021 Future Medicine Ltd.
Figures

References
-
- Ahmadyousefi Y, Malih S, Mirzaee Y, Saidijam M. Nucleic acid aptamers in diagnosis of colorectal cancer. Biochimie 156, 1–11 (2019). - PubMed
-
- Wolff JA, Malone RW, Williams P et al. Direct gene transfer into mouse muscle in vivo. Science 247(4949), 1465–1468 (1990). - PubMed
-
•• The first report that showed injection of naked plasmid DNA into mouse muscle results in a local expression of the transgene.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
