Single Dose of Bivalent H5 and H7 Influenza Virus-Like Particle Protects Chickens Against Highly Pathogenic H5N1 and H7N9 Avian Influenza Viruses
- PMID: 34859093
- PMCID: PMC8632145
- DOI: 10.3389/fvets.2021.774630
Single Dose of Bivalent H5 and H7 Influenza Virus-Like Particle Protects Chickens Against Highly Pathogenic H5N1 and H7N9 Avian Influenza Viruses
Abstract
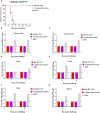
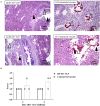
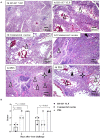
Both H5N1 and H7N9 subtype avian influenza viruses cause enormous economic losses and pose considerable threats to public health. Bivalent vaccines against both two subtypes are more effective in control of H5N1 and H7N9 viruses in poultry and novel egg-independent vaccines are needed. Herein, H5 and H7 virus like particle (VLP) were generated in a baculovirus expression system and a bivalent H5+H7 VLP vaccine candidate was prepared by combining these two antigens. Single immunization of the bivalent VLP or commercial inactivated vaccines elicited effective antibody immune responses, including hemagglutination inhibition, virus neutralizing and HA-specific IgG antibodies. All vaccinated birds survived lethal challenge with highly pathogenic H5N1 and H7N9 viruses. Furthermore, the bivalent VLP significantly reduced viral shedding and virus replication in chickens, which was comparable to that observed for the commercial inactivated vaccine. However, the bivalent VLP was better than the commercial vaccine in terms of alleviating pulmonary lesions caused by H7N9 virus infection in chickens. Therefore, our study suggests that the bivalent H5+H7 VLP vaccine candidate can serve as a critical alternative for the traditional egg-based inactivated vaccines against H5N1 and H7N9 avian influenza virus infection in poultry.
Keywords: H5N1 subtype; H7N9 subtype; avian influenza virus; baculovirus expression system; bivalent vaccine; chickens; virus-like particle.
Copyright © 2021 Hu, Peng, Li, Zhang, Li, Wang, Gu, Hu, Hu, Liu, Jiao, Peng and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
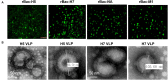
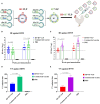
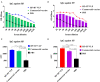
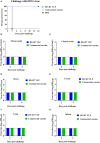
Similar articles
-
A Replication-Defective Influenza Virus Harboring H5 and H7 Hemagglutinins Provides Protection against H5N1 and H7N9 Infection in Mice.J Virol. 2021 Jan 13;95(3):e02154-20. doi: 10.1128/JVI.02154-20. Print 2021 Jan 13. J Virol. 2021. PMID: 33177192 Free PMC article.
-
H7N9 influenza virus-like particle based on BEVS protects chickens from lethal challenge with highly pathogenic H7N9 avian influenza virus.Vet Microbiol. 2021 Jul;258:109106. doi: 10.1016/j.vetmic.2021.109106. Epub 2021 May 8. Vet Microbiol. 2021. PMID: 34004568
-
Protective efficacy of an inactivated chimeric H7/H5 avian influenza vaccine against highly pathogenic avian influenza H7N9 and clade 2.3.4.4 H5 viruses.Vet Microbiol. 2018 Sep;223:21-26. doi: 10.1016/j.vetmic.2018.07.011. Epub 2018 Jul 17. Vet Microbiol. 2018. PMID: 30173747
-
Development and use of fowlpox vectored vaccines for avian influenza.Ann N Y Acad Sci. 2006 Oct;1081:193-201. doi: 10.1196/annals.1373.023. Ann N Y Acad Sci. 2006. PMID: 17135511 Review.
-
Innovation in Newcastle Disease Virus Vectored Avian Influenza Vaccines.Viruses. 2019 Mar 26;11(3):300. doi: 10.3390/v11030300. Viruses. 2019. PMID: 30917500 Free PMC article. Review.
Cited by
-
Respiratory Viruses and Virus-like Particle Vaccine Development: How Far Have We Advanced?Viruses. 2023 Jan 30;15(2):392. doi: 10.3390/v15020392. Viruses. 2023. PMID: 36851606 Free PMC article. Review.
-
Nanomaterial Adjuvants for Veterinary Vaccines: Mechanisms and Applications.Research (Wash D C). 2025 Jul 8;8:0761. doi: 10.34133/research.0761. eCollection 2025. Research (Wash D C). 2025. PMID: 40636132 Free PMC article. Review.
-
Virus-like particles in poultry disease: an approach to effective and safe vaccination.Front Vet Sci. 2024 Sep 9;11:1405605. doi: 10.3389/fvets.2024.1405605. eCollection 2024. Front Vet Sci. 2024. PMID: 39315089 Free PMC article. Review.
-
A single immunization with H5N1 virus-like particle vaccine protects chickens against divergent H5N1 influenza viruses and vaccine efficacy is determined by adjuvant and dosage.Emerg Microbes Infect. 2024 Dec;13(1):2287682. doi: 10.1080/22221751.2023.2287682. Epub 2023 Dec 30. Emerg Microbes Infect. 2024. PMID: 37994795 Free PMC article.
-
H3N2 canine influenza virus-like particle vaccine with great protection in beagle dogs.Microbiol Spectr. 2024 Jun 14;12(8):e0044524. doi: 10.1128/spectrum.00445-24. Online ahead of print. Microbiol Spectr. 2024. PMID: 38874403 Free PMC article.
References
-
- Imai M, Watanabe T, Kiso M, Nakajima N, Yamayoshi S, Iwatsuki-Horimoto K, et al. . A highly pathogenic avian H7N9 influenza virus isolated from a human is lethal in some ferrets infected via respiratory droplets. Cell Host Microbe. (2017) 22:615–26 e618. 10.1016/j.chom.2017.09.008 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

