Paclitaxel-based supramolecular hydrogel loaded with mifepristone for the inhibition of breast cancer metastasis
- PMID: 34859546
- PMCID: PMC8819302
- DOI: 10.1111/cas.15230
Paclitaxel-based supramolecular hydrogel loaded with mifepristone for the inhibition of breast cancer metastasis
Abstract
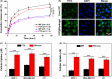
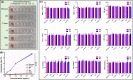
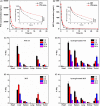
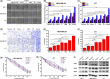
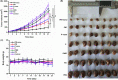
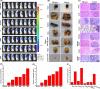
Breast cancer is the leading cause of cancer death among women and almost all of the breast cancer-caused mortality is related to metastasis. It has been reported that glucocorticoid facilitates the metastasis of breast cancer in mice, and mifepristone can antagonize the effect of glucocorticoid. Paclitaxel is one of the important drugs in the treatment of breast cancer. Mifepristone combined with paclitaxel could be an effective strategy for inhibiting breast cancer metastasis. However, their inherent defects, in terms of short blood circulation half-life and lack of tumor targeting, not only limit their effectiveness but also cause adverse reactions. Therefore, our aim is to explore a novel protocol against breast cancer metastasis, further optimize its therapeutic efficacy by a nanodelivery system, and explore its mechanism. Herein, a paclitaxel-conjugated and mifepristone-loaded hydrogel (PM-nano) was prepared by self-assembly. Its characterizations were studied. The antimetastatic effect was evaluated in vitro and in vivo and its mechanism was also explored by western blot assay. The resultant PM-nano was developed with favorable water solubility and good biocompatibility. Moreover, PM-nano displayed increased cell uptake properties and stimulated drug release in the tumor micro-acidic environment. The PM-nano was more effective in inhibiting the proliferation and metastasis of breast cancer than other groups in vitro and in vivo. The PM-nano might inhibit metastasis through glucocorticoid receptor/receptor tyrosine kinase-like orphan receptor 1 and MMPs. Taken together, PM-nano showed superior antimetastatic effects against breast cancer and excellent biocompatibility in vitro and in vivo, providing a new option for limiting metastasis.
Keywords: breast cancer; metastasis; mifepristone; paclitaxel; supramolecular hydrogel.
© 2021 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures

References
-
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209‐249. - PubMed
-
- Obradović MMS, Hamelin B, Manevski N, et al. Glucocorticoids promote breast cancer metastasis. Nature. 2019;567(7749):540‐544. - PubMed
MeSH terms
Substances
Grants and funding
- 18JCJQJC47300/National Science Fund for Distinguished Young Scholars of Tianjin
- 81701840/National Natural Science Foundation of China
- 2016-I2M-3-022/CAMS Innovation Fund for Medical Sciences
- 19ZXDBSY00090/Major Science and Technology Project for prevention and Treatment of major diseases in Tianjin
- 2018PT35031/Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences
LinkOut - more resources
Full Text Sources
Medical

