A Multi-enzyme Cascade for the Biosynthesis of AICA Ribonucleoside Di- and Triphosphate
- PMID: 34859954
- PMCID: PMC9299608
- DOI: 10.1002/cbic.202100596
A Multi-enzyme Cascade for the Biosynthesis of AICA Ribonucleoside Di- and Triphosphate
Abstract
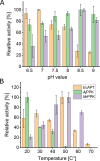
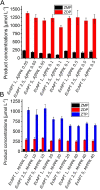
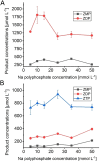
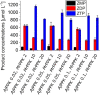
AICA (5'-aminoimidazole-4-carboxamide) ribonucleotides with different phosphorylation levels are the pharmaceutically active metabolites of AICA nucleoside-based drugs. The chemical synthesis of AICA ribonucleotides with defined phosphorylation is challenging and expensive. In this study, we describe two enzymatic cascades to synthesize AICA derivatives with defined phosphorylation levels from the corresponding nucleobase and the co-substrate phosphoribosyl pyrophosphate. The cascades are composed of an adenine phosphoribosyltransferase from Escherichia coli (EcAPT) and different polyphosphate kinases: polyphosphate kinase from Acinetobacter johnsonii (AjPPK), and polyphosphate kinase from Meiothermus ruber (MrPPK). The role of the EcAPT is to bind the nucleobase to the sugar moiety, while the kinases are responsible for further phosphorylation of the nucleotide to produce the desired phosphorylated AICA ribonucleotide. The selected enzymes were characterized, and conditions were established for two enzymatic cascades. The diphosphorylated AICA ribonucleotide derivative ZDP, synthesized from the cascade EcAPT/AjPPK, was produced with a conversion up to 91 %. The EcAPT/MrPPK cascade yielded ZTP with conversion up to 65 % with ZDP as a side product.
Keywords: adenine phosphoribosyltransferase; enzymatic nucleotide synthesis; enzyme cascades; nucleotide analogues; polyphosphate kinases.
© 2021 The Authors. ChemBioChem published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Figures


References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

