Concomitant genetic defects potentiate the adverse impact of prenatal alcohol exposure on cardiac outflow tract maturation
- PMID: 34859965
- PMCID: PMC10033225
- DOI: 10.1002/bdr2.1968
Concomitant genetic defects potentiate the adverse impact of prenatal alcohol exposure on cardiac outflow tract maturation
Abstract
Background: Prenatal alcohol exposure (PAE) is associated with an increased incidence of congenital heart defects (CHD), in particular outflow tract (OFT) defects. However, the variability in the incidence of CHD following PAE has not been fully explored. We hypothesize that a concomitant, relevant genetic defect would potentiate the adverse effect of PAE and partially explain the variability of PAE-induced CHD incidence.
Methods: The OFT is formed by the second heart field (SHF). Our PAE model consisted of two intraperitoneal injections (3 g/kg, separated by 6 hr) of 30% ethanol on E6.5 during SHF specification. The impact of genetic defects was studied by SHF-specific loss of Delta-like ligand 4 (Dll4), fibroblast growth factor 8 (Fgf8) and Islet1.
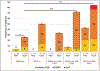
Results: Acute PAE alone significantly increased CHD incidence (4% vs. 26%, p = .015) with a particular increase in OFT alignment defects, viz., double outlet right ventricle (0 vs. 9%, p = .02). In embryos with a SHF genetic defect, acute PAE significantly increased CHD incidence (14 vs. 63%, p < .001), including double outlet right ventricle (6 vs. 50%, p < .001) compared to controls. PAE (p = .01) and heterozygous loss of Dll4 (p = .04) were found to independently contribute to CHD incidence, while neither Islet1 nor Fgf8 defects were found to be significant.
Conclusions: Our model recapitulates the increased incidence of OFT alignment defects seen in the clinic due to PAE. The presence of a concomitant SHF genetic mutation increases the incidence of PAE-related OFT defects. An apparent synergistic interaction between PAE and the loss of DLL4-mediated Notch signaling in OFT alignment requires further analysis.
Keywords: Notch; cardiac outflow tract; congenital heart defect; prenatal alcohol.
© 2021 Wiley Periodicals LLC.
Conflict of interest statement
CONFLICTS OF INTEREST
The authors declare no potential conflicts of interest.
Figures


Similar articles
-
Murine Model of Cardiac Defects Observed in Adams-Oliver Syndrome Driven by Delta-Like Ligand-4 Haploinsufficiency.Stem Cells Dev. 2021 Jun 15;30(12):611-621. doi: 10.1089/scd.2021.0058. Epub 2021 May 31. Stem Cells Dev. 2021. PMID: 33899511 Free PMC article.
-
Delta-like ligand 4-mediated Notch signaling controls proliferation of second heart field progenitor cells by regulating Fgf8 expression.Development. 2020 Sep 11;147(17):dev185249. doi: 10.1242/dev.185249. Development. 2020. PMID: 32778568 Free PMC article.
-
Rac1 Signaling Is Required for Anterior Second Heart Field Cellular Organization and Cardiac Outflow Tract Development.J Am Heart Assoc. 2015 Dec 31;5(1):e002508. doi: 10.1161/JAHA.115.002508. J Am Heart Assoc. 2015. PMID: 26722124 Free PMC article.
-
Prenatal alcohol exposure induced congenital heart diseases: From bench to bedside.Birth Defects Res. 2021 Apr 15;113(7):521-534. doi: 10.1002/bdr2.1743. Epub 2020 Jun 24. Birth Defects Res. 2021. PMID: 32578335 Review.
-
On the involvement of the second heart field in congenital heart defects.C R Biol. 2024 Mar 15;347:9-18. doi: 10.5802/crbiol.151. C R Biol. 2024. PMID: 38488639 Review.
Cited by
-
Molecular Pathways and Animal Models of Tetralogy of Fallot and Double Outlet Right Ventricle.Adv Exp Med Biol. 2024;1441:645-659. doi: 10.1007/978-3-031-44087-8_37. Adv Exp Med Biol. 2024. PMID: 38884739 Review.
-
Mutations in genes related to myocyte contraction and ventricular septum development in non-syndromic tetralogy of Fallot.Front Cardiovasc Med. 2023 Sep 28;10:1249605. doi: 10.3389/fcvm.2023.1249605. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37840956 Free PMC article.
-
Molecular genetic mechanisms of congenital heart disease.Curr Opin Genet Dev. 2022 Aug;75:101949. doi: 10.1016/j.gde.2022.101949. Epub 2022 Jul 8. Curr Opin Genet Dev. 2022. PMID: 35816939 Free PMC article. Review.
References
-
- Burd L, Deal E, Rios R, Adickes E, Wynne J, & Klug MG (2007). Congenital heart defects and fetal alcohol spectrum disorders. Congenital Heart Disease, 2, 250–255. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

