Features of Inflammatory Heart Reactions Following mRNA COVID-19 Vaccination at a Global Level
- PMID: 34860360
- PMCID: PMC9015432
- DOI: 10.1002/cpt.2499
Features of Inflammatory Heart Reactions Following mRNA COVID-19 Vaccination at a Global Level
Abstract
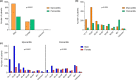
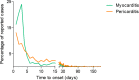
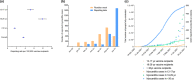
Myocarditis and pericarditis may constitute adverse reactions of mRNA coronavirus disease 2019 (COVID-19) vaccines. This study aimed to document these reactions and to assess the association with patient sex and age. This is as an observational retrospective study using a case-non-case design (also called disproportionality study) on inflammatory heart reactions reported with mRNA COVID-19 vaccines within the World Health Organization (WHO) global safety database (VigiBase), up to June 30, 2021. Results are expressed using reporting odds ratios (RORs) and their 95% confidence interval (95% CI). Of 716,576 reports related to mRNA COVID-19 vaccines, 2,277 were cases of inflammatory heart reactions, including 1241 (55%) myocarditis and 851 (37%) pericarditis. The main age group was 18-29 years (704, 31%), and mostly male patients (1,555, 68%). Pericarditis onset was delayed compared with myocarditis with a median time to onset of 8 (3-21) vs. 3 (2-6) days, respectively (P = 0.001). Regarding myocarditis, an important disproportionate reporting was observed in adolescents (ROR, 22.3, 95% CI 19.2-25.9) and in 18-29 years old (ROR, 6.6, 95% CI 5.9-7.5) compared with older patients, as well as in male patients (ROR, 9.4, 95% CI 8.3-10.6). Reporting rate of myocarditis was increased in young adults and adolescents. Inflammatory heart reactions may rarely occur shortly following mRNA COVID-19 vaccination. Although an important disproportionate reporting of myocarditis was observed among adolescents and young adults, particularly in male patients, reporting rates support a very rare risk, that does not seem to compromise the largely positive benefit-risk balance of these vaccines. Furthermore, this study confirmed the value of disproportionality analyses for estimation of relative risks among subgroups of patients.
© 2021 The Authors. Clinical Pharmacology & Therapeutics published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declared no competing interests for this work.
Figures
References
-
- Eckart, R.E. et al. Incidence and follow‐up of inflammatory cardiac complications after smallpox vaccination. J. Am. Coll. Cardiol. 44, 201–205 (2004). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

