Effectiveness of T cell-mediated rejection therapy: A systematic review and meta-analysis
- PMID: 34860468
- PMCID: PMC9300092
- DOI: 10.1111/ajt.16907
Effectiveness of T cell-mediated rejection therapy: A systematic review and meta-analysis
Abstract
The effectiveness of T cell-mediated rejection (TCMR) therapy for achieving histological remission remains undefined in patients on modern immunosuppression. We systematically identified, critically appraised, and summarized the incidence and histological outcomes after TCMR treatment in patients on tacrolimus (Tac) and mycophenolic acid (MPA). English-language publications were searched in MEDLINE (Ovid), Embase (Ovid), Cochrane Central (Ovid), CINAHL (EBSCO), and Clinicaltrials.gov (NLM) up to January 2021. Study quality was assessed with the National Institutes of Health Study Quality Tool. We pooled results using an inverse variance, random-effects model and report the binomial proportions with associated 95% confidence intervals (95% CI). Statistical heterogeneity was explored using the I2 statistic. From 2875 screened citations, we included 12 studies (1255 participants). Fifty-eight percent were good/high quality while the rest were moderate quality. Thirty-nine percent of patients (95% CI 0.26-0.53, I2 77%) had persistent ≥Banff Borderline TCMR 2-9 months after anti-rejection therapy. Pulse steroids and augmented maintenance immunosuppression were mainstays of therapy, but considerable practice heterogeneity was present. A high proportion of biopsy-proven rejection exists after treatment emphasizing the importance of histology to characterize remission. Anti-rejection therapy is foundational to transplant management but well-designed clinical trials in patients on Tac/MPA immunosuppression are lacking to define the optimal therapeutic approach.
Keywords: clinical research/practice; graft survival; immunosuppression/immune modulation; immunosuppressive regimens; kidney (allograft) function/dysfunction; kidney transplantation/nephrology; rejection: T cell mediated (TCMR); rejection: antibody-mediated (ABMR).
© 2021 The Authors. American Journal of Transplantation published by Wiley Periodicals LLC on behalf of The American Society of Transplantation and the American Society of Transplant Surgeons.
Figures




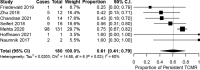


Comment in
-
T cell-mediated rejection in kidney transplant recipients: The end(point) is also the beginning.Am J Transplant. 2022 Mar;22(3):683-684. doi: 10.1111/ajt.16964. Epub 2022 Jan 28. Am J Transplant. 2022. PMID: 35073440 No abstract available.
References
-
- Nankivell BJ, Borrows RJ, Fung CL, O'Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349(24):2326‐2333. - PubMed
-
- Nickerson P, Jeffery J, Gough J, et al. Identification of clinical and histopathologic risk factors for diminished renal function 2 years posttransplant. J Am Soc Nephrol. 1998;9(3):482‐487. - PubMed
-
- Grimm PC, Nickerson P, Gough J, et al. Computerized image analysis of Sirius Red‐stained renal allograft biopsies as a surrogate marker to predict long‐term allograft function. J Am Soc Nephrol. 2003;14(6):1662‐1668. - PubMed
-
- Cosio FG, Grande JP, Wadei H, Larson TS, Griffin MD, Stegall MD. Predicting subsequent decline in kidney allograft function from early surveillance biopsies. Am J Transplant. 2005;5(10):2464‐2472. - PubMed
-
- Cosio FG, El Ters M, Cornell LD, Schinstock CA, Stegall MD. Changing kidney allograft histology early posttransplant: prognostic implications of 1‐year protocol biopsies. Am J Transplant. 2016;16(1):194‐203. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

