Mass Spectrometry Imaging of N-Linked Glycans in a Formalin-Fixed Paraffin-Embedded Human Prostate by Infrared Matrix-Assisted Laser Desorption Electrospray Ionization
- PMID: 34860526
- PMCID: PMC9944006
- DOI: 10.1021/acs.jproteome.1c00822
Mass Spectrometry Imaging of N-Linked Glycans in a Formalin-Fixed Paraffin-Embedded Human Prostate by Infrared Matrix-Assisted Laser Desorption Electrospray Ionization
Abstract
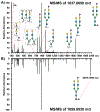
N-Linked glycans are structurally diverse polysaccharides that represent significant biological relevance due to their involvement in disease progression and cancer. Due to their complex nature, N-linked glycans pose many analytical challenges requiring the continued development of analytical technologies. Infrared matrix-assisted laser desorption electrospray ionization (IR-MALDESI) is a hybrid ionization technique commonly used for mass spectrometry imaging (MSI) applications. Previous work demonstrated IR-MALDESI to significantly preserve sialic acid containing N-linked glycans that otherwise require chemical derivatization prior to detection. Here, we demonstrate the first analysis of N-linked glycans in situ by IR-MALDESI MSI. A formalin-fixed paraffin-embedded human prostate tissue was analyzed in negative ionization mode after tissue washing, antigen retrieval, and pneumatic application of PNGase F for enzymatic digestion of N-linked glycans. Fifty-three N-linked glycans were confidently identified in the prostate sample where more than 60% contained sialic acid residues. This work demonstrates the first steps in N-linked glycan imaging of biological tissues by IR-MALDESI MSI. Raw data files are available in MassIVE (identifier: MSV000088414).
Keywords: IR-MALDESI; N-linked glycans; mass spectrometry imaging; prostate cancer.
Figures




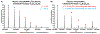
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

