Daytime eating prevents internal circadian misalignment and glucose intolerance in night work
- PMID: 34860550
- PMCID: PMC8641939
- DOI: 10.1126/sciadv.abg9910
Daytime eating prevents internal circadian misalignment and glucose intolerance in night work
Abstract
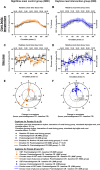
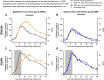
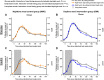
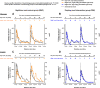
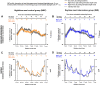
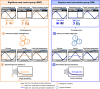
Night work increases diabetes risk. Misalignment between the central circadian “clock” and daily behaviors, typical in night workers, impairs glucose tolerance, likely due to internal misalignment between central and peripheral circadian rhythms. Whether appropriate circadian alignment of eating can prevent internal circadian misalignment and glucose intolerance is unknown. In a 14-day circadian paradigm, we assessed glycemic control during simulated night work with either nighttime or daytime eating. Assessment of central (body temperature) and peripheral (glucose and insulin) endogenous circadian rhythms happened during constant routine protocols before and after simulated night work. Nighttime eating led to misalignment between central and peripheral (glucose) endogenous circadian rhythms and impaired glucose tolerance, whereas restricting meals to daytime prevented it. These findings offer a behavioral approach to preventing glucose intolerance in shift workers.
Figures


References
-
- NHIS, 2010 National Health Interview Survey. Public-use data file and documentation (2010); ftp.cdc.gov/pub/Health_Statistics/NCHS/Dataset_Documentation/NHIS/2010/s... [accessed 19 February 2020].
-
- A. Parent-Thirion, I. Biletta, J. Cabrita, O. Vargas Llave, G. Vermeylen, A. Wilczyńska, M. Wilkens, Sixth European Working Conditions Survey—Overview report (2019); www.eurofound.europa.eu/publications/report/2016/working-conditions/sixt... [accessed 19 February 2020].
-
- Kroenke C. H., Spiegelman D., Manson J., Schernhammer E. S., Colditz G. A., Kawachi I., Work characteristics and incidence of type 2 diabetes in women. Am. J. Epidemiol. 165, 175–183 (2007). - PubMed
-
- Manodpitipong A., Saetung S., Nimitphong H., Siwasaranond N., Wongphan T., Sornsiriwong C., Luckanajantachote P., Mangjit P., Keesukphan P., Crowley S. J., Hood M. M., Reutrakul S., Night-shift work is associated with poorer glycaemic control in patients with type 2 diabetes. J. Sleep Res. 26, 764–772 (2017). - PubMed
Grants and funding
- R01 HL118601/HL/NHLBI NIH HHS/United States
- R01 HL140577/HL/NHLBI NIH HHS/United States
- K24 HL103845/HL/NHLBI NIH HHS/United States
- R01 DK102696/DK/NIDDK NIH HHS/United States
- R01 HL140574/HL/NHLBI NIH HHS/United States
- UL1 TR001102/TR/NCATS NIH HHS/United States
- R01 HL125893/HL/NHLBI NIH HHS/United States
- R01 DK099512/DK/NIDDK NIH HHS/United States
- UL1 TR002541/TR/NCATS NIH HHS/United States
- R01 HL142064/HL/NHLBI NIH HHS/United States
- K99 HL148500/HL/NHLBI NIH HHS/United States
- R01 DK105072/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

