A particulate saponin/TLR agonist vaccine adjuvant alters lymph flow and modulates adaptive immunity
- PMID: 34860581
- PMCID: PMC8763571
- DOI: 10.1126/sciimmunol.abf1152
A particulate saponin/TLR agonist vaccine adjuvant alters lymph flow and modulates adaptive immunity
Abstract
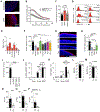
Saponins are potent and safe vaccine adjuvants, but their mechanisms of action remain incompletely understood. Here, we explored the properties of several saponin formulations, including immune-stimulatory complexes (ISCOMs) formed by the self-assembly of saponin and phospholipids in the absence or presence of the Toll-like receptor 4 agonist monophosphoryl lipid A (MPLA). We found that MPLA self-assembles with saponins to form particles physically resembling ISCOMs, which we termed saponin/MPLA nanoparticles (SMNP). Saponin-containing adjuvants exhibited distinctive mechanisms of action, altering lymph flow in a mast cell–dependent manner and promoting antigen entry into draining lymph nodes. SMNP was particularly effective, exhibiting even greater potency than the compositionally related adjuvant AS01B in mice, and primed robust germinal center B cell, TFH, and HIV tier 2 neutralizing antibodies in nonhuman primates. Together, these findings shed new light on mechanisms by which saponin adjuvants act to promote the immune response and suggest that SMNP may be a promising adjuvant in the setting of HIV, SARS-CoV-2, and other pathogens.
Figures






References
-
- Rao M, Alving CR, Adjuvants for HIV vaccines. Current Opinion in HIV and AIDS 11, 585–592 (2016). - PubMed
-
- Noe AR, Kotraiah V, Gutierrez GM, Enabling Vaccine Delivery Platforms and Adjuvants for Malaria. Current Topics in Malaria, 387 (2016).
-
- Arunachalam PS, Walls AC, Golden N, Atyeo C, Fischinger S, Li C, Aye P, Navarro MJ, Lai L, Edara VV, Roltgen K, Rogers K, Shirreff L, Ferrell DE, Wrenn S, Pettie D, Kraft JC, Miranda MC, Kepl E, Sydeman C, Brunette N, Murphy M, Fiala B, Carter L, White AG, Trisal M, Hsieh CL, Russell-Lodrigue K, Monjure C, Dufour J, Spencer S, Doyle-Meyers L, Bohm RP, Maness NJ, Roy C, Plante JA, Plante KS, Zhu A, Gorman MJ, Shin S, Shen X, Fontenot J, Gupta S, O'Hagan DT, Van Der Most R, Rappuoli R, Coffman RL, Novack D, McLellan JS, Subramaniam S, Montefiori D, Boyd SD, Flynn JL, Alter G, Villinger F, Kleanthous H, Rappaport J, Suthar MS, King NP, Veesler D, Pulendran B, Adjuvanting a subunit COVID-19 vaccine to induce protective immunity. Nature 594, 253–258 (2021). - PubMed
-
- Lvgren Bengtsson K, Morein B, Osterhaus ADME, ISCOM technology-based Matrix M\texttrademark adjuvant: success in future vaccines relies on formulation. Expert review of vaccines 10, 401–403 (2011). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI153098/AI/NIAID NIH HHS/United States
- UM1 AI144462/AI/NIAID NIH HHS/United States
- UM1 AI100663/AI/NIAID NIH HHS/United States
- T32 AI007386/AI/NIAID NIH HHS/United States
- R61 AI161818/AI/NIAID NIH HHS/United States
- P30 CA014051/CA/NCI NIH HHS/United States
- P01 AI048240/AI/NIAID NIH HHS/United States
- R01 CA214913/CA/NCI NIH HHS/United States
- P01 AI104715/AI/NIAID NIH HHS/United States
- U42 OD011023/OD/NIH HHS/United States
- R61 AI161297/AI/NIAID NIH HHS/United States
- R01 AI137057/AI/NIAID NIH HHS/United States
- P51 OD011132/OD/NIH HHS/United States
- R01 AI125068/AI/NIAID NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R37 AI125068/AI/NIAID NIH HHS/United States
- P51 OD011133/OD/NIH HHS/United States
- S10 OD021831/OD/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

