The infection risks of JAK inhibition
- PMID: 34860621
- PMCID: PMC8935945
- DOI: 10.1080/1744666X.2022.2014323
The infection risks of JAK inhibition
Abstract
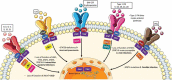
Introduction: Janus Kinase inhibitors (JAKi) have shown to be highly effective in the treatment of immune-mediated inflammatory diseases. As with all immunomodulatory therapies, careful assessment of any treatment-associated infection risk is essential to inform clinical decision-making.
Areas covered: We summarize current literature on infection rates among the licensed JAKi using published phase II/III trial results, post-licensing and registry data.
Expert opinion: licensed JAKi show increased risk of infection across the class compared to placebo, most commonly affecting respiratory and urinary tracts, nasopharynx and skin. This risk is dose-dependent. Risks are similar at licensed JAKi doses to that seen with biologic therapies. The risk is compounded by other risk factors for infection, such as age and steroid co-prescription. Herpes zoster reactivation is more common with JAKi compared to other targeted immune modulation, making screening for varicella exposure and vaccination in appropriate cohorts an advisable strategy. Crucially, these small risk increases must be balanced against the known harms (including infection) of uncontrolled autoimmune disease. JAKi are a safe and potentially transformative treatment when used for appropriately selected patients.
Keywords: Adults; Adverse events; JAK inhibitors; inflammatory disease; older adults; serious infections; tuberculosis; vaccination; varicella zoster.
Figures

References
-
- Murray PJ. The JAK-STAT signaling pathway: input and output integration. J Immunol. Epub 2007/ 02/22. PubMed PMID: 17312100 2007;178(5):2623–2629. - PubMed
-
- Kisseleva T, Bhattacharya S, Braunstein J, et al. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene. 2002;285(1–2):1–24. Epub 2002/ 06/01. PubMed PMID: 12039028 - PubMed
-
- Winthrop KL. The emerging safety profile of JAK inhibitors in rheumatic disease. Nat Rev Rheumatol. 2017;13(4):234–243. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
