The FDA Funding Crisis
- PMID: 34861005
- PMCID: PMC6005411
- DOI: 10.1177/8755122513505224
The FDA Funding Crisis
Abstract
The role of the Food and Drug Administration (FDA) is to ensure the safety of prescription and nonprescription drugs, dietary supplements, and the food supply, representing more than 20% of US consumer spending. The increased need to monitor imported drugs, drug products and foods, drug shortages, and compounding pharmacies bring the adequacy of FDA funding into question. Performing even at status quo cannot be accomplished if responsibilities increase without equitable funding increases: both from the federal government and fees imposed on FDA-regulated industries. Additionally, scientific advancement, new legislation, and new industries are continually increasing the FDA workload, necessitating commensurate budget increases.
Keywords: FDA; drug compounding; funding; quality control; regulation; safety.
© The Author(s) 2013.
Conflict of interest statement
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
Similar articles
-
Potential risks of pharmacy compounding.Drugs R D. 2013 Mar;13(1):1-8. doi: 10.1007/s40268-013-0005-9. Drugs R D. 2013. PMID: 23526368 Free PMC article. Review.
-
FDA Approval and Regulation of Pharmaceuticals, 1983-2018.JAMA. 2020 Jan 14;323(2):164-176. doi: 10.1001/jama.2019.20288. JAMA. 2020. PMID: 31935033
-
The history and contemporary challenges of the US Food and Drug Administration.Clin Ther. 2007 Jan;29(1):1-16. doi: 10.1016/j.clinthera.2007.01.006. Clin Ther. 2007. PMID: 17379043
-
The importance of GRAS to the functional food and nutraceutical industries.Toxicology. 2006 Apr 3;221(1):17-27. doi: 10.1016/j.tox.2006.01.012. Epub 2006 Feb 17. Toxicology. 2006. PMID: 16483705
-
Complementary and integrative medical therapies, the FDA, and the NIH: definitions and regulation.Dermatol Ther. 2003;16(2):77-84. doi: 10.1046/j.1529-8019.2003.01614.x. Dermatol Ther. 2003. PMID: 12919107 Review.
References
-
- Hamburg MA. President’s Fiscal Year 2014 budget request for the FDA. http://www.fda.gov/NewsEvents/Testimony/ucm351980.htm. Published April, 26, 2013. Accessed June 15, 2013.
-
- Elder D. Ensuring the safety of imported products. http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm048631.htm. Published 2010. Accessed June 15, 2013.
-
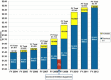
- Alliance for a Stronger FDA. FDA funding FY 2004 to FY 2012. 2013. http://fdaalliance.files.wordpress.com/2009/11/picture11.png. Published 2013. Accessed June 15, 2013.
-
- Moore TJ, Psaty BM, Furberg CD. Time to act on drug safety. JAMA. 1998;279:1571-1573. - PubMed
-
- Cohen PA. Assessing supplement safety—the FDA’s controversial proposal. N Engl J Med. 2012;366:389-391. - PubMed
LinkOut - more resources
Full Text Sources