Direct comparison of antibody responses to four SARS-CoV-2 vaccines in Mongolia
- PMID: 34861167
- PMCID: PMC8585611
- DOI: 10.1016/j.chom.2021.11.004
Direct comparison of antibody responses to four SARS-CoV-2 vaccines in Mongolia
Abstract
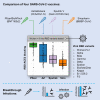
Different SARS-CoV-2 vaccines are approved in various countries, but few direct comparisons of the antibody responses they stimulate have been reported. We collected plasma specimens in July 2021 from 196 Mongolian participants fully vaccinated with one of four COVID-19 vaccines: Pfizer/BioNTech, AstraZeneca, Sputnik V, and Sinopharm. Functional antibody testing with a panel of nine SARS-CoV-2 viral variant receptor binding domain (RBD) proteins revealed marked differences in vaccine responses, with low antibody levels and RBD-ACE2 blocking activity stimulated by the Sinopharm and Sputnik V vaccines in comparison to the AstraZeneca or Pfizer/BioNTech vaccines. The Alpha variant caused 97% of infections in Mongolia in June and early July 2021. Individuals who recover from SARS-CoV-2 infection after vaccination achieve high antibody titers in most cases. These data suggest that public health interventions such as vaccine boosting, potentially with more potent vaccine types, may be needed to control COVID-19 in Mongolia and worldwide.
Keywords: COVID-19; Mongolia; Pfizer/BioNTech; SARS-CoV-2; Sinopharm; Sputnik V; serology; vaccine; viral variants.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests S.D.B. has consulted for Regeneron, Sanofi, and Novartis on topics unrelated to this study and owns stock in AbCellera Biologics; B.P. and P.S.A. are inventors on a provisional patent application (no. 63/026,577) submitted by the Board of Trustees of the Leland Stanford Junior University, Stanford, CA, that covers the use of “Therapeutic Methods for Treating COVID-19 Infections”; B.P. serves on the External Immunology Network of GSK and on the Scientific Advisory Board of Medicago and Boehringer Ingelheim; and K.C.N. reports grants from National Institute of Allergy and Infectious Diseases (NIAID), Food Allergy Research & Education (FARE), End Allergies Together (EAT), National Heart, Lung, and Blood Institute (NHLBI), and National Institute of Environmental Health Sciences (NIEHS). K.C.N. is Director of FARE and World Allergy Organization (WAO) Center of Excellence at Stanford, Advisor at Cour Pharmaceuticals, Cofounder of Before Brands, Alladapt, Latitude, and IgGenix, National Scientific Committee member for the Immune Tolerance Network (ITN) of NIAID, recipient of a Research Sponsorship from Nestle, Consultant and Advisory Board Member at Before Brands, Alladapt, IgGenix, NHLBI, and ProBio, and Data and Safety Monitoring Board member at NHLBI; R.S. Chinthrajah receives grant support from CoFAR National Institute of Allergy and Infectious Diseases, Aimmune, DBV Technologies, Astellas, AnaptysBio, Novartis, and Regeneron and is an advisory board member for Alladapt Immunotherapeutics Inc., Novartis, and Genentech. J.L.W., J.N.W., and G.B.S. are employees of Meso Scale Diagnostics (MSD). The rest of the authors declare that they have no conflicts of interest relevant to this manuscript.
Figures

References
-
- AlQahtani M., Bhattacharyya S., Alawadi A., Mahmeed H.A., Sayed J.A., Justman J., El-Sadr W.M., Hidary J., Mukherjee S. Research Square; 2021. Morbidity and mortality from COVID-19 post-vaccination breakthrough infections in association with vaccines and the emergence of variants in Bahrain. - DOI
-
- Davies N.G., Abbott S., Barnard R.C., Jarvis C.I., Kucharski A.J., Munday J.D., Pearson C.A.B., Russell T.W., Tully D.C., Washburne A.D., et al. CMMID COVID-19 Working Group. COVID-19 Genomics UK (COG-UK) Consortium Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021;372:eabg3055. - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

