Safety and efficacy of the mRNA BNT162b2 vaccine against SARS-CoV-2 in five groups of immunocompromised patients and healthy controls in a prospective open-label clinical trial
- PMID: 34861491
- PMCID: PMC8629680
- DOI: 10.1016/j.ebiom.2021.103705
Safety and efficacy of the mRNA BNT162b2 vaccine against SARS-CoV-2 in five groups of immunocompromised patients and healthy controls in a prospective open-label clinical trial
Abstract
Background: Patients with immunocompromised disorders have mainly been excluded from clinical trials of vaccination against COVID-19. Thus, the aim of this prospective clinical trial was to investigate safety and efficacy of BNT162b2 mRNA vaccination in five selected groups of immunocompromised patients and healthy controls.
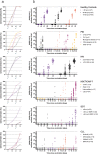
Methods: 539 study subjects (449 patients and 90 controls) were included. The patients had either primary (n=90), or secondary immunodeficiency disorders due to human immunodeficiency virus infection (n=90), allogeneic hematopoietic stem cell transplantation/CAR T cell therapy (n=90), solid organ transplantation (SOT) (n=89), or chronic lymphocytic leukemia (CLL) (n=90). The primary endpoint was seroconversion rate two weeks after the second dose. The secondary endpoints were safety and documented SARS-CoV-2 infection.
Findings: Adverse events were generally mild, but one case of fatal suspected unexpected serious adverse reaction occurred. 72.2% of the immunocompromised patients seroconverted compared to 100% of the controls (p=0.004). Lowest seroconversion rates were found in the SOT (43.4%) and CLL (63.3%) patient groups with observed negative impact of treatment with mycophenolate mofetil and ibrutinib, respectively.
Interpretation: The results showed that the mRNA BNT162b2 vaccine was safe in immunocompromised patients. Rate of seroconversion was substantially lower than in healthy controls, with a wide range of rates and antibody titres among predefined patient groups and subgroups. This clinical trial highlights the need for additional vaccine doses in certain immunocompromised patient groups to improve immunity.
Funding: Knut and Alice Wallenberg Foundation, the Swedish Research Council, Nordstjernan AB, Region Stockholm, Karolinska Institutet, and organizations for PID/CLL-patients in Sweden.
Keywords: CAR-T; HIV; Immunocompromised patients; Primary Immunodeficiency; chronic lymphocytic leukemia; human stem-cell transplantation; mRNA BNT162b2 vaccine; solid organ transplantation.
Copyright © 2021 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest SM received honoraria via his institution from Celgene/BMS, Novartis, Gilead/Kite, DNA Prime for lectures and educational events and as a member and/or head of data safety monitoring boards from Miltenyi and Immunicum outside the submitted work. SH has been taking part in a COVID-19 Strategic Consultancy Group and a Virtual Advisory Board, not related to the current study. KL reports grants from Knut and Alice Wallenberg Foundation for this study. HGL reports grants from Knut and Alice Wallenberg Foundation and Nordstjernan AB for studies on COVID-19, and has served on the UK-CIC Oversight Committee, is leading the Karolinska Institutet COVID-19 vaccine group, and has served on several Karolinska Institutet COVID-19 Task force and Reference groups. PL reports grants from Pfizer, grants from MSD, grants and personal fees from Takeda, personal fees from AiCuris, personal fees from OctaPharma, outside the submitted work. SA has received honoraria for lectures and educational events, not related to this work, from Gilead, AbbVie, MSD, Biogen and Netdoktor, and reports grants from Knut and Alice Wallenberg Foundation for this study.
Figures


References
-
- Kim JS, Lee KH, Kim GE, et al. Clinical characteristics and mortality of patients with hematologic malignancies and COVID-19: a systematic review. Eur Rev Med Pharmacol Sci. 2020;24(22):11926–11933. - PubMed
-
- Ljungman P, Mikulska M, de la Camara R, et al. The challenge of COVID-19 and hematopoietic cell transplantation; EBMT recommendations for management of hematopoietic cell transplant recipients, their donors, and patients undergoing CAR T-cell therapy. Bone Marrow Transplant. 2020;55(11):2071–2076. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

