Altered fibrin clot structure and dysregulated fibrinolysis contribute to thrombosis risk in severe COVID-19
- PMID: 34861681
- PMCID: PMC8648369
- DOI: 10.1182/bloodadvances.2021004816
Altered fibrin clot structure and dysregulated fibrinolysis contribute to thrombosis risk in severe COVID-19
Abstract
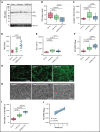
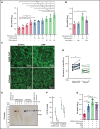
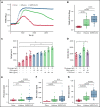
The high incidence of thrombotic events suggests a possible role of the contact system pathway in COVID-19 pathology. In this study, we determined the altered levels of factor XII (FXII) and its activation products in critically ill patients with COVID-19 in comparison with patients with severe acute respiratory distress syndrome related to the influenza virus (acute respiratory distress syndrome [ARDS]-influenza). Compatible with those data, we found rapid consumption of FXII in COVID-19 but not in ARDS-influenza plasma. Interestingly, the lag phase in fibrin formation, triggered by the FXII activator kaolin, was not prolonged in COVID-19, as opposed to that in ARDS-influenza. Confocal and electron microscopy showed that increased FXII activation rate, in conjunction with elevated fibrinogen levels, triggered formation of fibrinolysis-resistant, compact clots with thin fibers and small pores in COVID-19. Accordingly, clot lysis was markedly impaired in COVID-19 as opposed to that in ARDS-influenza. Dysregulated fibrinolytic system, as evidenced by elevated levels of thrombin-activatable fibrinolysis inhibitor, tissue-plasminogen activator, and plasminogen activator inhibitor-1 in COVID-19 potentiated this effect. Analysis of lung tissue sections revealed widespread extra- and intravascular compact fibrin deposits in patients with COVID-19. A compact fibrin network structure and dysregulated fibrinolysis may collectively contribute to a high incidence of thrombotic events in COVID-19.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

