Targeting the ATM Kinase to Enhance the Efficacy of Radiotherapy and Outcomes for Cancer Patients
- PMID: 34861994
- PMCID: PMC8647772
- DOI: 10.1016/j.semradonc.2021.09.008
Targeting the ATM Kinase to Enhance the Efficacy of Radiotherapy and Outcomes for Cancer Patients
Abstract
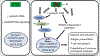
Targeting the DNA damage response represents a promising approach to improve the efficacy of radiation therapy. One appealing target for this approach is the serine/threonine kinase ataxia telangiectasia mutated (ATM), which is activated by DNA double strand breaks to orchestrate the cellular response to ionizing radiation. Small-molecule inhibitors targeting ATM have entered clinical trials testing their safety in combination with radiation therapy or in combination with other DNA damaging agents. Here, we review biochemical, genetic, and cellular functional studies of ATM, phenotypes associated with germline and somatic cancer mutations in ATM in humans, and experiments in genetically engineered mouse models that support a rationale for investigating ATM inhibitors as radiosensitizers for cancer therapy. These data identify important synthetic lethal relationships, which suggest that ATM inhibitors may be particularly effective in tumors with defects in other nodes of the DNA damage response. The potential for ATM inhibition to improve immunotherapy responses in preclinical models represents another emerging area of research. We summarize ongoing clinical trials of ATM inhibitors with radiotherapy. We also discuss critical ongoing areas of investigation that include discovery of biomarkers that predict for radiosensitization by ATM inhibitors and identification of effective combinations of ATM inhibitors, radiation therapy, other DNA damage response-directed therapies, and/or immunotherapies.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest DGK is a co-founder of Xrad Therapeutics, which is developing radiosensitizers that target kinases including ATM, and serves on the Scientific Advisory Board of Lumicell, which is commercializing intraoperative imaging technology. DGK is a co-inventor on patents for radiosensitizers and an intraoperative imaging device. DGK also receives funding for a clinical trial from a Stand Up To Cancer (SU2C) Catalyst Research Grant with support from Merck. The laboratory of DGK currently receives funding or reagents from Merck, Amgen, Bristol-Myers Squibb, Varian Medical Systems, and Calithera, but these did not support this manuscript. ZJR is a co-inventor on patents related to cancer diagnostic tests that are managed by Duke University and have been licensed to Genetron Health.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

