Nutrient Sensor mTORC1 Regulates Insulin Secretion by Modulating β-Cell Autophagy
- PMID: 34862201
- PMCID: PMC8893949
- DOI: 10.2337/db21-0281
Nutrient Sensor mTORC1 Regulates Insulin Secretion by Modulating β-Cell Autophagy
Abstract
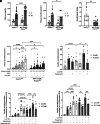
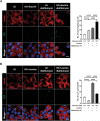
The dynamic regulation of autophagy in β-cells by cycles of fasting-feeding and its effects on insulin secretion are unknown. In β-cells, mechanistic target of rapamycin complex 1 (mTORC1) is inhibited while fasting and is rapidly stimulated during refeeding by a single amino acid, leucine, and glucose. Stimulation of mTORC1 by nutrients inhibited the autophagy initiator ULK1 and the transcription factor TFEB, thereby preventing autophagy when β-cells were continuously exposed to nutrients. Inhibition of mTORC1 by Raptor knockout mimicked the effects of fasting and stimulated autophagy while inhibiting insulin secretion, whereas moderate inhibition of autophagy under these conditions rescued insulin secretion. These results show that mTORC1 regulates insulin secretion through modulation of autophagy under different nutritional situations. In the fasting state, autophagy is regulated in an mTORC1-dependent manner, and its stimulation is required to keep insulin levels low, thereby preventing hypoglycemia. Reciprocally, stimulation of mTORC1 by elevated leucine and glucose, which is common in obesity, may promote hyperinsulinemia by inhibiting autophagy.
© 2022 by the American Diabetes Association.
Figures






References
-
- Mizushima N. Autophagy in protein and organelle turnover. Cold Spring Harb Symp Quant Biol 2011;76:397–402 - PubMed
-
- Jung HS, Chung KW, Won Kim J, et al. Loss of autophagy diminishes pancreatic beta cell mass and function with resultant hyperglycemia. Cell Metab 2008;8:318–324 - PubMed
-
- Ebato C, Uchida T, Arakawa M, et al. Autophagy is important in islet homeostasis and compensatory increase of beta cell mass in response to high-fat diet. Cell Metab 2008;8:325–332 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

