A low aldosterone/renin ratio and high soluble ACE2 associate with COVID-19 severity
- PMID: 34862332
- PMCID: PMC8815849
- DOI: 10.1097/HJH.0000000000003054
A low aldosterone/renin ratio and high soluble ACE2 associate with COVID-19 severity
Abstract
Background: The severity of COVID-19 after SARS-CoV-2 infection is unpredictable. Angiotensin-converting enzyme-2 (ACE2) is the receptor responsible for coronavirus binding, while subsequent cell entry relies on priming by the serine protease TMPRSS2 (transmembrane protease, serine 2). Although renin-angiotensin-aldosterone-system (RAAS) blockers have been suggested to upregulate ACE2, their use in COVID-19 patients is now considered well tolerated. The aim of our study was to investigate parameters that determine COVID-19 severity, focusing on RAAS-components and variation in the genes encoding for ACE2 and TMPRSS2.
Methods: Adult patients hospitalized due to SARS-CoV-2 infection between May 2020 and October 2020 in the Haga Teaching Hospital were included, and soluble ACE2 (sACE2), renin, aldosterone (in heparin plasma) and polymorphisms in the ACE2 and TMPRSS2 genes (in DNA obtained from EDTA blood) were determined.
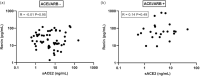
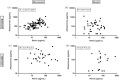
Measurements and main results: Out of the 188 patients who were included, 60 were defined as severe COVID-19 (ICU and/or death). These patients more often used antidiabetic drugs, were older, had higher renin and sACE2 levels, lower aldosterone levels and a lower aldosterone/renin ratio. In addition, they displayed the TMPRSS2-rs2070788 AA genotype less frequently. No ACE2 polymorphism-related differences were observed. Multivariate regression analysis revealed independent significance for age, sACE2, the aldosterone/renin ratio, and the TMPRSS2 rs2070788 non-AA genotype as predictors of COVID-19 severity, together yielding a C-index of 0.79. Findings were independent of the use of RAAS blockers.
Conclusion: High sACE2, a low aldosterone/renin ratio and having the TMPRSS2 rs2070788 non-AA genotype are novel independent determinants that may help to predict COVID-19 disease severity.
Trial registration: retrospectively registered.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The ethics review board of The Hague Hospital (number T20-054) approved the study protocol, and written informed consent was obtained from all participants after enrolment. This prospective observational study has made use of data as part of the International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC). All patients who were hospitalized with a confirmed SARS-CoV-2 infection and of whom residual blood samples were available, were eligible for participation by opt-out consent.
Exclusion occurred in case patients were pregnant or transported to another hospital, if residual blood was absent, or in case of a rejection of previously inclusion by opt-out consent.
The authors report no conflicts of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

