Prenatal administration of IL-1Ra attenuate the neurodevelopmental impacts following non-pathogenic inflammation during pregnancy
- PMID: 34862457
- PMCID: PMC8642433
- DOI: 10.1038/s41598-021-02927-3
Prenatal administration of IL-1Ra attenuate the neurodevelopmental impacts following non-pathogenic inflammation during pregnancy
Abstract
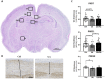
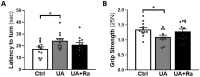
Prenatal inflammation negatively affects placental function, subsequently altering fetal development. Pathogen-associated molecular patterns (PAMPs) are used to mimics infections in preclinical models but rarely detected during pregnancy. Our group previously developed an animal model of prenatal exposure to uric acid (endogenous mediator), leading to growth restriction alongside IL-1-driven placental inflammation (Brien et al. in J Immunol 198(1):443-451, 2017). Unlike PAMPs, the postnatal impact of prenatal non-pathogenic inflammation is still poorly understood. Therefore, we investigated the effects of prenatal uric acid exposure on postnatal neurodevelopment and the therapeutic potential of the IL-1 receptor antagonist; IL-1Ra. Uric acid induced growth restriction and placental inflammation, which IL-1Ra protected against. Postnatal evaluation of both structural and functional aspects of the brain revealed developmental changes. Both astrogliosis and microgliosis were observed in the hippocampus and white matter at postnatal day (PND)7 with IL-1Ra being protective. Decreased myelin density was observed at PND21, and reduced amount of neuronal precursor cells was observed in the Dentate Gyrus at PND35. Functionally, motor impairments were observed as evaluated with the increased time to fully turn upward (180 degrees) on the inclined plane and the pups were weaker on the grip strength test. Prenatal exposure to sterile inflammation, mimicking most clinical situation, induced growth restriction with negative impact on neurodevelopment. Targeted anti-inflammatory intervention prenatally could offer a strategy to protect brain development during pregnancy.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Uric Acid Crystals Induce Placental Inflammation and Alter Trophoblast Function via an IL-1-Dependent Pathway: Implications for Fetal Growth Restriction.J Immunol. 2017 Jan 1;198(1):443-451. doi: 10.4049/jimmunol.1601179. Epub 2016 Nov 30. J Immunol. 2017. PMID: 27903743 Free PMC article.
-
Postnatal administration of IL-1Ra exerts neuroprotective effects following perinatal inflammation and/or hypoxic-ischemic injuries.Brain Behav Immun. 2012 Nov;26(8):1331-9. doi: 10.1016/j.bbi.2012.09.001. Epub 2012 Sep 13. Brain Behav Immun. 2012. PMID: 22982341 Free PMC article.
-
Maternal Inflammation Disrupts Fetal Neurodevelopment via Increased Placental Output of Serotonin to the Fetal Brain.J Neurosci. 2016 Jun 1;36(22):6041-9. doi: 10.1523/JNEUROSCI.2534-15.2016. J Neurosci. 2016. PMID: 27251625 Free PMC article.
-
Nicotine protects fetus against LPS-induced fetal growth restriction through ameliorating placental inflammation and vascular development in late pregnancy in rats.Biosci Rep. 2019 Jul 2;39(7):BSR20190386. doi: 10.1042/BSR20190386. Print 2019 Jul 31. Biosci Rep. 2019. PMID: 31209145 Free PMC article.
-
Antenatal Suppression of IL-1 Protects against Inflammation-Induced Fetal Injury and Improves Neonatal and Developmental Outcomes in Mice.J Immunol. 2017 Mar 1;198(5):2047-2062. doi: 10.4049/jimmunol.1601600. Epub 2017 Feb 1. J Immunol. 2017. PMID: 28148737
Cited by
-
Animal Models of Chorioamnionitis: Considerations for Translational Medicine.Biomedicines. 2022 Mar 30;10(4):811. doi: 10.3390/biomedicines10040811. Biomedicines. 2022. PMID: 35453561 Free PMC article. Review.
-
Fever in childbirth: a mini-review of epidural-related maternal fever.Front Neurosci. 2024 Apr 19;18:1389132. doi: 10.3389/fnins.2024.1389132. eCollection 2024. Front Neurosci. 2024. PMID: 38707593 Free PMC article. Review.
-
The Role of the Interleukin-1 Family in Complications of Prematurity.Int J Mol Sci. 2023 Feb 1;24(3):2795. doi: 10.3390/ijms24032795. Int J Mol Sci. 2023. PMID: 36769133 Free PMC article. Review.
-
Complex Neuroimmune Involvement in Neurodevelopment: A Mini-Review.J Inflamm Res. 2023 Jul 19;16:2979-2991. doi: 10.2147/JIR.S410562. eCollection 2023. J Inflamm Res. 2023. PMID: 37489149 Free PMC article. Review.
-
Cross-Generational Impact of Innate Immune Memory Following Pregnancy Complications.Cells. 2022 Dec 6;11(23):3935. doi: 10.3390/cells11233935. Cells. 2022. PMID: 36497193 Free PMC article. Review.
References
-
- Girard S, et al. IL-1 receptor antagonist protects against placental and neurodevelopmental defects induced by maternal inflammation. J. Immunol. 2010;184(7):3997–4005. - PubMed
-
- Hagberg H, Gressens P, Mallard C. Inflammation during fetal and neonatal life: Implications for neurologic and neuropsychiatric disease in children and adults. Ann. Neurol. 2012;71(4):444–457. - PubMed
-
- Allard MJ, et al. A sexually dichotomous, autistic-like phenotype is induced by Group B Streptococcus maternofetal immune activation. Autism Res. 2017;10(2):233–245. - PubMed
-
- Allard MJ, et al. Causal role of group B Streptococcus-induced acute chorioamnionitis in intrauterine growth retardation and cerebral palsy-like impairments. J. Dev. Orig. Health Dis. 2019;10(5):595–602. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

